¿Qué es Triadas de Dobereiner? » Su Definición y Significado [2022]
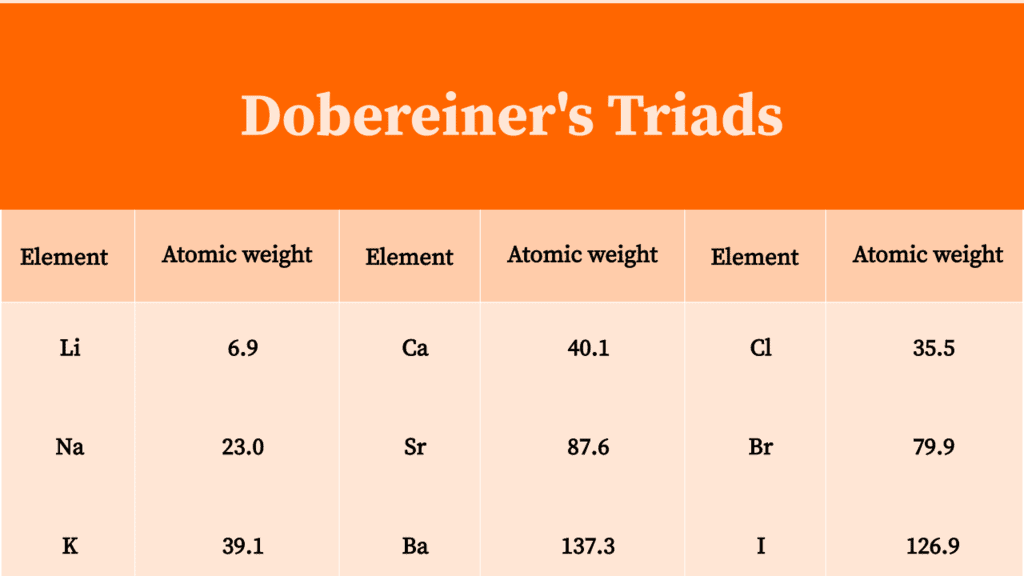
The Dobereiner's triad's examples: Lithium- 6.94. Sodium- 22.99. Potassium- 39.1. Thus the atomic masses give the arithmetic means that corresponds to 23.02 and are also similar to the atomic mass of sodium. Triad 2: Calcium, strontium, and barium are some strong examples of the Law of triads. Barium- 137.3,

Döbereiner's Triads School Of Elements Part 2 YouTube
Join us on a journey into the history of chemistry! Discover the fascinating concept of Dobereiner's Triads, a groundbreaking idea by 19th-century chemist Jo.

Dobereiner`s law of triads stock illustration. Illustration of periodic 250967359
Hello Friends, Checkout our Video on Explanation Video Dobereiner's Triads0:00 Introduction0:10 Dobereiner's Triads1:05 Dobereiner's law of Traids 1:53 1.

Dobereiner's Triads Classification, Chemistry Lecture Sabaq.pk YouTube
Johann Wolfgang Dobereiner, who attempted to sort the elements in an order which consisted of triads. In the history of the periodic table, Dobereiner's triads were an early attempt to sort the elements into some logical order by their physical properties. In 1817, a letter reported Johann Wolfgang Dobereiner's observations of the alkaline.

Dobereiner's Triads Periodic table Chemistry Class 11 YouTube
One of the most famous examples of a Dobereiner Triad is the alkali metal triad: lithium (Li), sodium (Na), and potassium (K). These elements share similar chemical properties, and their atomic weights form a clear pattern: the atomic weight of sodium is approximately the mean of lithium and potassium. This kind of relationship was also evident.

Trick to learn Doberenier Triad / dobereiner's law of triads / NEET chemistry video lecturer
This was known as the Dobereiner's law of triads. The law states that when elements are placed in the ascending order of atomic masses, groups of three elements having similar properties are obtained. The atomic mass of the middle element of the triad is equal to the mean of the atomic masses of the other two elements of the triad.

Dobereiner's Triads Newland Law of Octaves Periodic Classification of Elements ClassX
By 1829, Döbereiner had found other groups of three elements (hence "triads") whose physical properties were similarly related. He also noted that some quantifiable properties of elements (e.g. atomic weight and density) in a triad followed a trend whereby the value of the middle element in the triad would be exactly or nearly predicted by taking the arithmetic mean of values for that.

Periodic Classification of Elements part 1 Dobereiner's Triads, Newland law of Octaves YouTube
← Previous page Dobereiners's Triads Johann Wolfgang Dobereiner was a German chemist. His effort is considered as one of the earliest attempts to classify the elements into groups. The earliest classification categorized elements into metals and non-metals. It was difficult to classify the elements, such as boron, which exhibited the properties of both metals as […]

Dobereiner's Law Of Triads In Just 4 Minutes Chemistry Calibre Tutorial YouTube
Quick Reference. A set of triads of chemically similar elements noted by Johann Döbereiner (1780-1849) in 1817. Even with the inaccurate atomic mass data of the day it was observed that when each triad was arranged in order of increasing atomic mass, then the mass of the central member was approximately the average of the values for the.

Dobereiner's Triads Class 10 Chemistry Periodic Classification of Elements YouTube
The first of Dobereiner's triads was identified in the year 1817 and was constituted by the alkaline earth metals calcium, strontium and barium. Three more triads were identified by the year 1829. These triads are tabulated below. Triad 1. This triad was made up of the alkali metals lithium, sodium and potassium.

Classification of elements//dobereiner's law of triads// YouTube
A triad is a grouping of three elements in order of atomic mass. Dobereiner's Law of Triads deals with putting three elements in order of atomic weight. The average of the first and third elements.

Dobereiner triads SCIENCE EVOLUTION
Hence they form Dobereiner's triad. Question 6: State the alkaline earth metal Dobereiner's triad. Answer: The alkaline earth metals that are a part of the Dobereiner triads are calcium, strontium and barium. Question 7: Verify the law of triads for the alkali metals triad. Answer: The alkali metals triad consists of Lithium. Sodium and.

Dobereiner's Triads Chemistry YouTube
Johann Wolfgang Dobereiner. As the number of elements increased, chemists inevitably began to find patterns in their properties.. that the atomic weight of the middle element in each triad is about equal to the average of the atomic weights of the first and third elements. The atomic weight of sodium (22.99 g/mol), for example, is remarkably.

Dobereiner's Triads 02 YouTube
In 1817, the first Dobereiner's triad was discovered. Calcium, strontium, and barium are alkaline earth metals that make up this compound. Three more triads were identified later on. Let's take a closer look at these triads. The following are five of Dobereiner's triads: Triad 1: The alkali metals lithium, sodium, and potassium made up.
Dobereiner's Triads, Newlands Octaves and Mendeleev's Periodic Table Class 11 Notes EduRev
furfural. magnesium. triad. Johann Wolfgang Döbereiner (born Dec. 13, 1780, Hof an der Saale [Germany]—died March 24, 1849, Jena) was a German chemist whose observation of similarities among certain elements anticipated the development of the periodic system of elements. As a coachman's son, Döbereiner had little opportunity for formal.

Na=Li+K/2=7+39/2=46/2=23 What is dobereiners law of triads explain with the help of one example
Elements X , Y , and Z belong to Dobereiner's triad. The atomic masses of X and Z are as follows. The atomic mass of the element Y is roughly equal to . Stuck? Use a hint. Learn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance, history, and more. Khan Academy is a nonprofit with the.