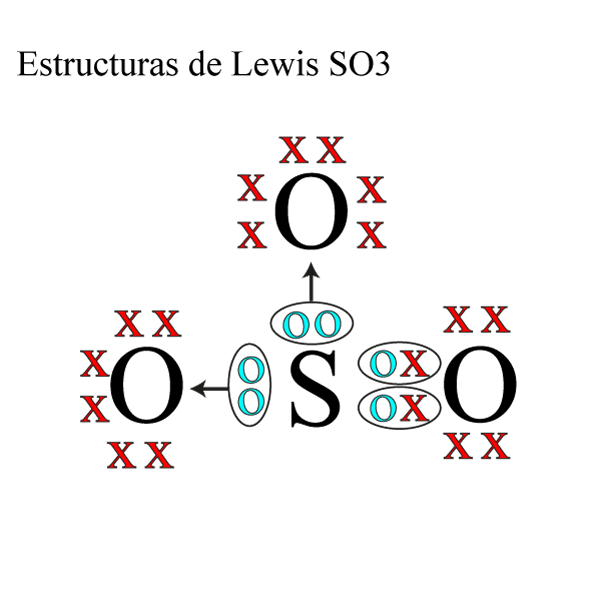
Estructura de Lewis SO3 » Quimica Online
DIFFERENTIAL EQUATIONS LW.Knowles and R.T. Lewis (Editors) 0 Elsevier Science Publishers B.V. (North-Holland), 1984 7 ON POSITIVE SOLUTIONS OF ELLIPTIC EQUATIONS WITH PERIODIC COEFFICIENTS IN En,SPECTRAL RESULTS AND EXTENSIONS TO ELLIPTIC OPERATORS ON RIEMANNIAN MANIFOLDS Institute of Mathematics Hebrew University of Jerusalem Jerusalem, Israel Shmuel Agmon 1.
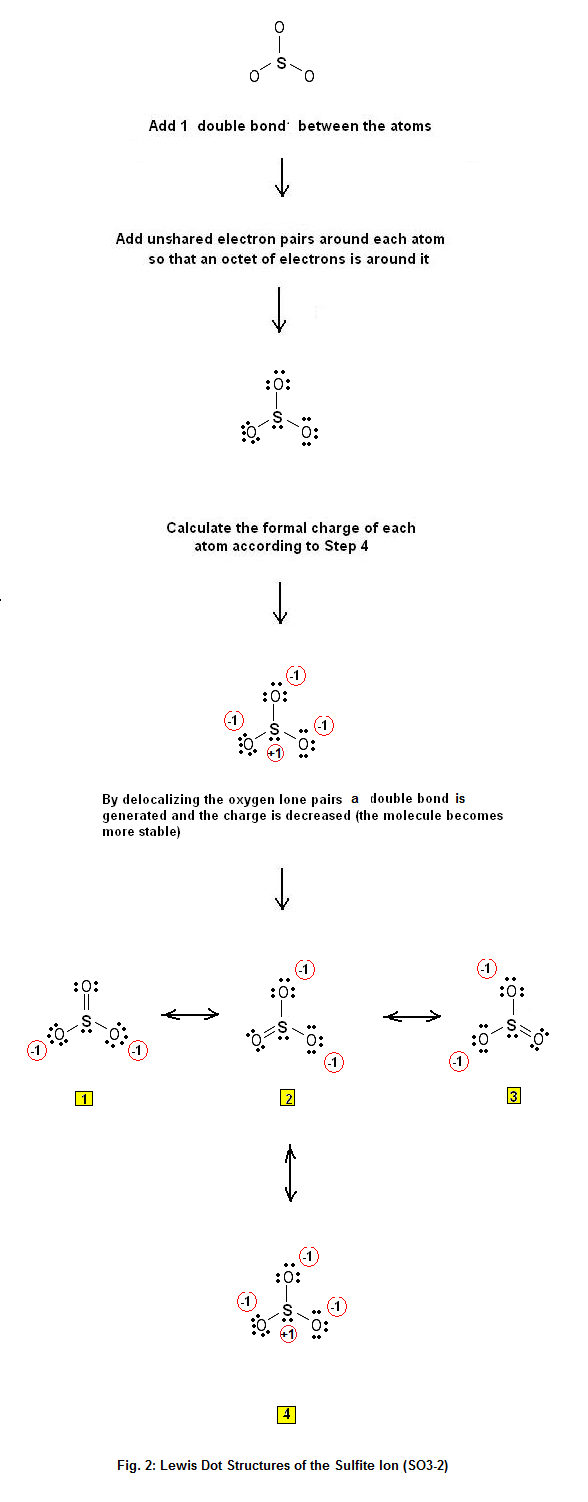
Lewis Dot Structure of the sulfite ion SO32 Electron Dot Structure Chemistry Net
Lewis Structure Finder. This widget gets the Lewis structure of chemical compounds. Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.

SO3 Lewis Structure, Molecular Geometry, and Hybridization Techiescientist
Sulfur trioxide (alternative spelling sulphur trioxide, also known as nisso sulfan) is the chemical compound with the formula SO 3.It has been described as "unquestionably the most [economically important]" sulfur oxide. It is prepared on an industrial scale as a precursor to sulfuric acid.. Sulfur trioxide exists in several forms - gaseous monomer, crystalline trimer, and solid polymer.

Calculating SO3 Formal Charges Calculating Formal Charges for SO3 YouTube
Website-http://www.kentchemistry.com/links/bonding/LewisDotTutorials/SO3.htmI quickly take you through how to draw the Lewis Structure of SO3 (Sulfur Trioxid.

How to draw SO3 Lewis Structure? Science Education and Tutorials
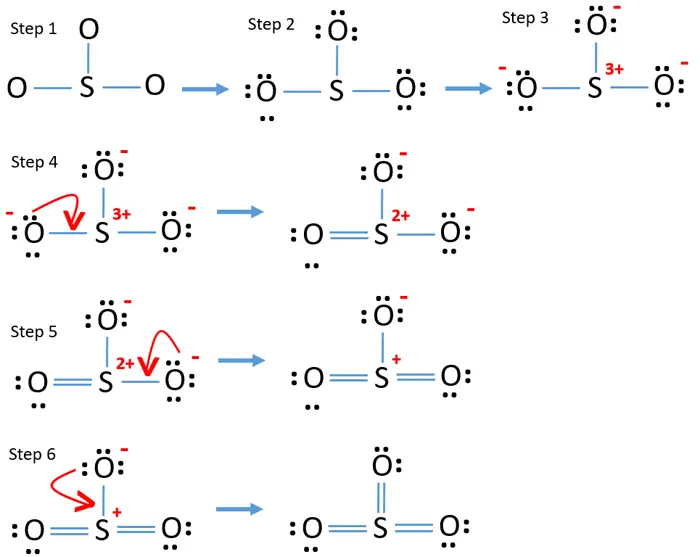
The SO3 Lewis structure shows a central Sulfur (S) atom with three Oxygen (O) atoms around it. These atoms are connected by double bonds, and each Oxygen atom has two lone pairs of electrons. In this page, you'll find a detailed, step-by-step guide on how to draw the Lewis structure for SO3. Step-by-Step Guide to Drawing the Lewis Structure.

So3 Lewis Structure
Lewis structure of SO 3 molecule. There are three double bonds around sulfur atom with oxygen atoms in SO molecule. Each oxygen atom has two lone pairs in SO 3 lewis structure. But, there is no lone pair on sulfur atom in SO 3 lewis structure as lewis structure of SO 2.. Hybridization of SO 3 molecule. All atoms have sp 2 hybridization. Each oxygen atom has one sigma bond and two lone pairs.

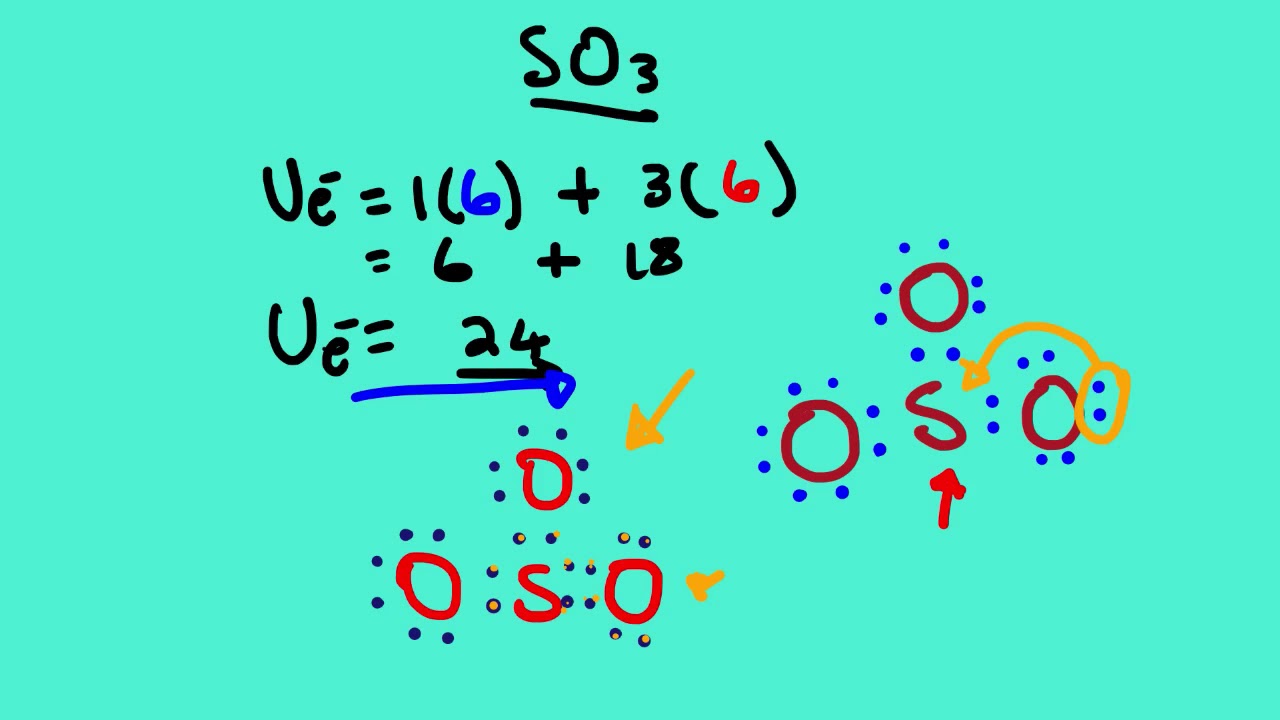
So far, we’ve used 24 of the SO3 Lewis structure’s total 24 outermost valence shell electrons
Step #1: Calculate the total number of valence electrons. Here, the given molecule is SO3 (sulfur trioxide). In order to draw the lewis structure of SO3, first of all you have to find the total number of valence electrons present in the SO3 molecule. (Valence electrons are the number of electrons present in the outermost shell of an atom).
Lewis Dot Diagram For So3 Wiring Diagram
The SO3 Lewis structure illustrates how the atoms of sulfur trioxide, a molecule composed of one sulfur atom and three oxygen atoms, are arranged. Within the SO 3 Lewis structure, the sulfur atom is bonded to three oxygen atoms through double bonds. Additionally, each oxygen atom has two lone pairs of electrons associated with it.
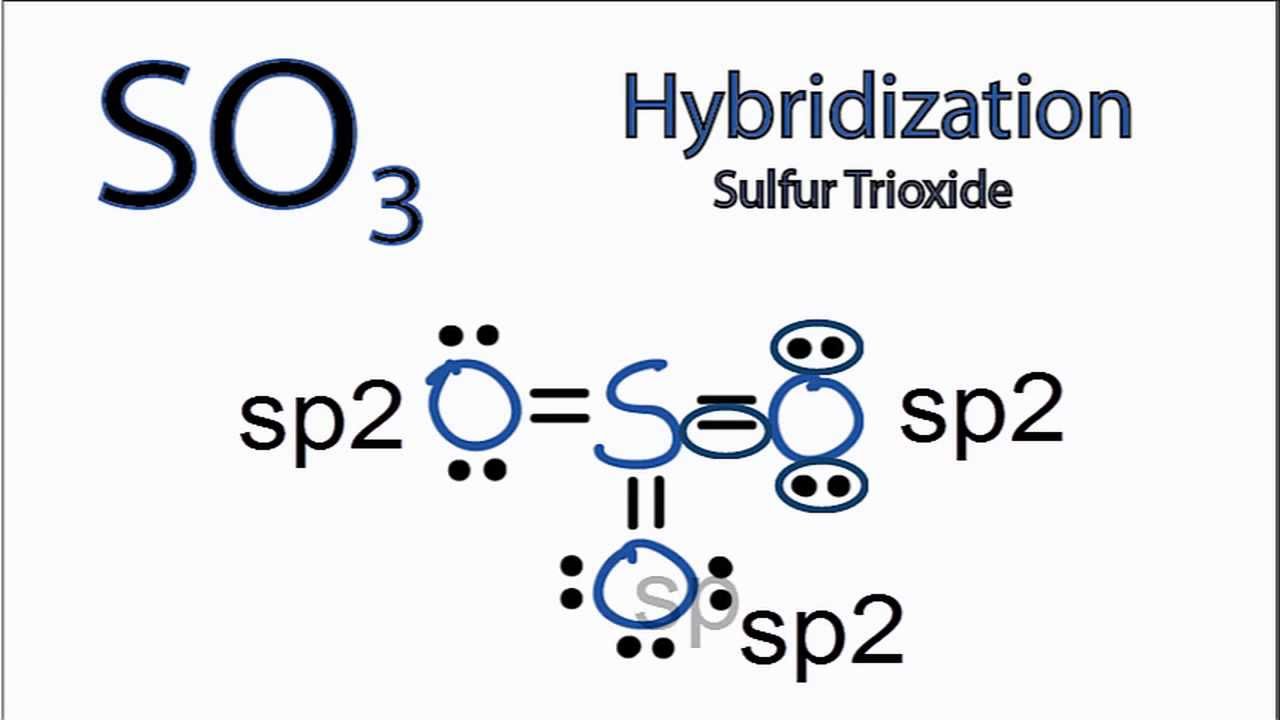
SO3 Hybridization Hybrid Orbitals for SO3 (sulfur trioxide) YouTube
Molecular geometry is the three-dimensional structure of the atoms which helps in the constitution of a molecule. It can determine reactivity, polarity, color, attraction, biological activity, etc. SO3 includes two components mainly - Sulfur and Oxygen. There are one sulfur atom and three oxygen atoms which are spread out as far away as they can!

Lewis Dot Structure for SO3 (Sulfur trioxide) YouTube
Drawing the Lewis Structure for SO 3 ( Sulfur Trioxide) SO 3 is the primary contributer to acid rain in the atomsphere. It is a form of pollution. SO 3 is named Sulfur Trioxide. There are 32 valence electrons available for the Lewis structure for SO 3. Be sure to check the formal charges for the Lewis structure for SO 3 .

Lewis Dot Structure For So3 slidesharedocs
This chemistry video explains how to draw the Lewis structure of SO3 - Sulfur Trioxide. It discusses the molecular geometry, bond angle, hybridization, and.
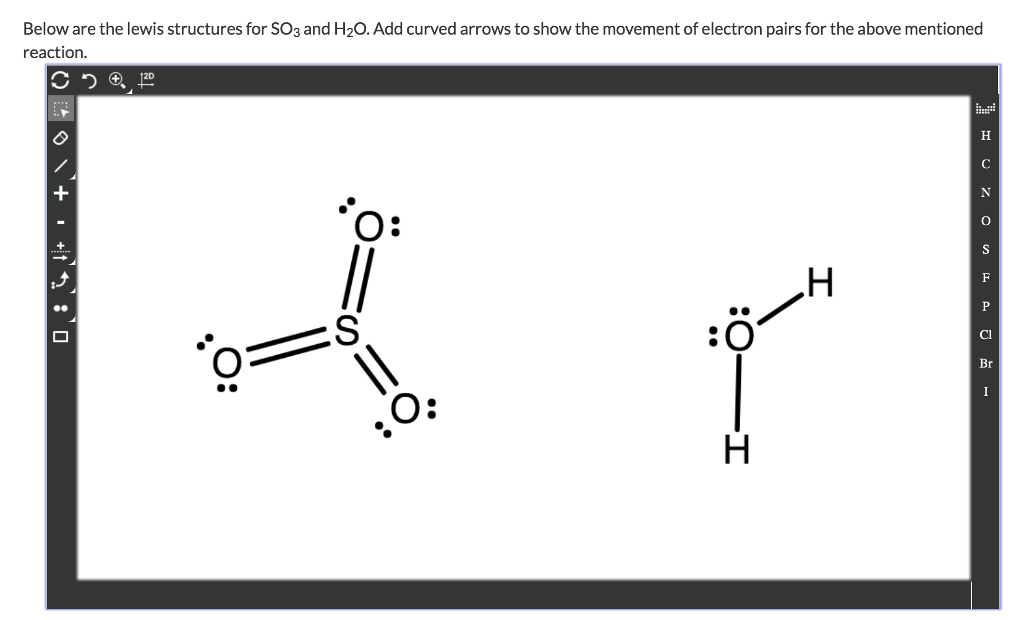
So3 Lewis Structure Angles
A step-by-step explanation of how to draw the SO3 Lewis Dot Structure (Sulfur trioxide).For the SO3 structure use the periodic table to find the total number.
steps of drawing SO3 lewis structure VSEPR method
A uniform asymptotic analysis of dispersive wave motion. D. S. Ahluwalia E. Reiss S. Stone. Physics. 1974. The signaling problem for the one dimensional Klein-Gordon equation with spatially varying coefficients is analyzed. A formal, uniformly valid, asymptotic expansion of the solution is obtained with…. Expand.
Estrutura De Lewis So3 MATERILEA
Lewis Structures. Page ID. A Lewis Structure is a very simplified representation of the valence shell electrons in a molecule. It is used to show how the electrons are arranged around individual atoms in a molecule. Electrons are shown as "dots" or for bonding electrons as a line between the two atoms. The goal is to obtain the "best" electron.

Estrutura De Lewis So3
Lewis Structure. The formal charge of an atom in a Lewis structure is a way to determine the distribution of electrons and the stability of the molecule. To calculate the formal charge, we use the formula:. Formal Charge = Valence Electrons - Lone Pairs - 1/2 * Bonded Electrons. In the SO3 Lewis structure, the formal charge of the sulfur atom is 0, while each oxygen atom has a formal.

SO3 2 Lewis Structure How to Draw the Lewis Structure for SO3 2 (Sulfite Ion) YouTube
How to draw the Lewis Structure of SO3 (sulfur trioxide) - with explanationSulfur is an exception to the octet rule - it can handle up to 12 electrons!Check.