
Cara Menghitung Ph Larutan Asam Kuat Dan Asam Lemah Panduan Kimia Riset Sexiz Pix
These are real scientific discoveries about the nature of the human body, which can be invaluable to physicians taking care of patients. Have feedback about this calculator? The Serum Osmolality/Osmolarity calculates expected serum osmolarity, for comparison to measured osmolarity to detect unmeasured compounds in the serum.

Gula Darah Keluarga Pengertian Diabetes
Molar concentration (also called molarity, amount concentration or substance concentration) is a measure of the concentration of a chemical species, in particular, of a solute in a solution, in terms of amount of substance per unit volume of solution. In chemistry, the most commonly used unit for molarity is the number of moles per liter, having the unit symbol mol/L or mol/dm 3 in SI units.

Rumus Mol (5)
More information from the unit converter. How many mmol in 1 mol? The answer is 1000. We assume you are converting between millimole and mole.You can view more details on each measurement unit: mmol or mol The SI base unit for amount of substance is the mole. 1 mmol is equal to 0.001 mole. Note that rounding errors may occur, so always check the results.

Mg/Dl To Umol/L / treatment for kidney disease Creatinine 4.4 mg/ dL Enter the creatinine
Quick conversion chart of mole to mmol. 1 mole to mmol = 1000 mmol. 2 mole to mmol = 2000 mmol. 3 mole to mmol = 3000 mmol. 4 mole to mmol = 4000 mmol. 5 mole to mmol = 5000 mmol. 6 mole to mmol = 6000 mmol. 7 mole to mmol = 7000 mmol. 8 mole to mmol = 8000 mmol.

Mengenal Rumus Keliling Lingkaran Dan Contoh Soalnya Blog Teknisi Mobile Legends
HOMA-IR = (insulin × glucose) / 22.5. for the glucose concentration in mmol/L, or: HOMA-IR = (insulin × glucose ) / 405. for glycemia in mg/dL. In both cases, the insulin is in mU/L. It is also worth noting that diabetes mellitus type 2 always coexists with insulin resistance (as insulin resistance is a background for the disease), so there.
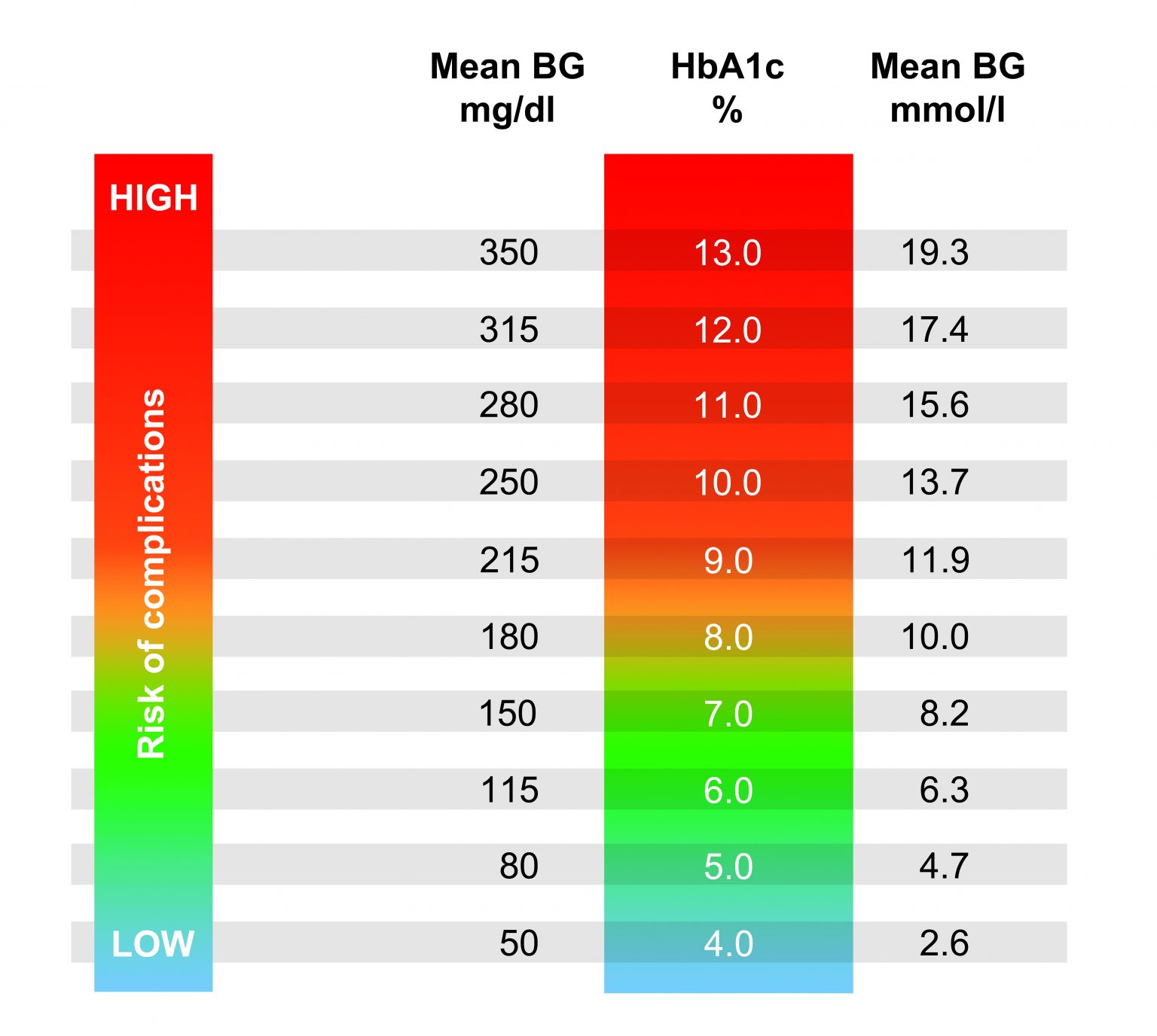
Normal Blood Sugar 1 Hour After Eating Mmol
Mmol is a measure of amount of substance. Get more information and details on the 'mmol' measurement unit, including its symbol, category, and common conversions from mmol to other amount of substance units. Measurement unit conversion: mmol. Measurement unit: mmol. Full name: millimole.

Rumus Osmolaritas Meteor
Rumus normalitas larutan adalah. N = ek/V atau N = n x a /V atau N = M x a. Keterangan: N = normalitas ( mol ek/L) V = volume larutan (liter) n = mol suatu zat (mol) a = ekivalen suatu zat. ek adalah mol ekivalen yaitu jumlah mol dikali jumlah ion H+ atau ion OH- Jika n mol zat terlarut mengandung sebanyak a ion H+ atau OH-, maka rumus mol.
Rumus Menghitung Volume Balok Format Soal Riset
eGFR Calculator. Glomerular filtration rate (GFR) is the best overall index of kidney function. Normal GFR varies according to age, sex, and body size, and declines with age. The National Kidney Foundation (NKF) and the American Society of Nephrology (ASN) convened a Task Force in 2021 to focus on the use of race when estimating GFR.

Fraksi mol dan molalitas (m) Soal dan Pembahasan KIMIA KELAS 12 YouTube
Contoh Soal Konsep Mol Beserta Jawabannya. 1. Sebanyak 4,9 gram H2SO4 H 2 SO 4 dilarutkan dalam air sehingga diperoleh 500 mL larutan. Tentukan jumlah ion yang yang terlarut dalam larutan dan tentukan konsentrasi ion H+ H +. Jawaban contoh soal konsep mol dan penyelesaiannya. nH2SO4 = m Mr = 4, 9 98 = 0, 05 mol n H 2 SO 4 = m M r = 4, 9 98 = 0.

Mmol/l là gì? Công thức tính mmol/l và Ứng dụng của nồng độ mol (8/2023)
mmol/l, µmol/l, mg/dl, mg/100ml, mg%, mg/l, µg/ml Creatinine is a breakdown product of creatine phosphate in muscle, and is usually produced at a fairly constant rate by the body (depending on muscle mass). It is freely filtered by the glomeruli and, under normal conditions, is not reabsorbed by the tubules to any appreciable extent.
Satuan Luas Pengertian Konversi Rumus Dan Contoh Soal Riset
Ingat, rumus molaritas pencampuran adalah: Maka, konsentrasi larutan setelah dicampurkan adalah: M campuran = (100 x 0,1) + (150 x 0,2) / (100 + 150) = 40 / 250. = 0,16 M. Quipperian, itu dia pembahasan mengenai rumus molaritas beserta contoh soal dan pembahasannya. Agar semakin paham dan mahir dalam menggunakan rumus molaritas, cobalah untuk.

mmol/L to mg/dl Conversion Share FUDiabetes
The mole (abbreviation mol) is the SI unit for the amount of a substance. A mmol (one thousandth of a mol) is a way to talk about a part of a mol. Thus, the conversion factor from mol to mmol is 1,000 mmol in 1 mol. Converting to mmol can be convenient if the number of mol is small.

Rumus Normalitas, Molaritas, Molalitas Pengertian, Rumus, Contoh Soal dan Pembahasan
Urea [mmol/L] x 1 = BUN [mmol/L] 1) To convert from mg/dL of blood urea nitrogen to mmol/L of urea, multiply by 0.357 (each molecule of urea having 2 nitrogens, each of molar mass 14g/mol) (BUN is the mass of nitrogen within urea/volume, not the mass of urea) Urea [mmol/L] = BUN [mg/dL of nitrogen] x 10 [dL/L] / 14x2 [mg N/mmol urea] (the mass.

berapa mmol CH2O yang ada dalam 45 ml suatu larutan dengan konsentrasi 0,4 M? Brainly.co.id
Baca Express tampilkan 1 Memahami Konsep Mmol 2 Cara Menghitung Mmol 3 Contoh Kasus Penghitungan Mmol 4 Kesimpulan Memahami Konsep Mmol Hello Kaum Berotak! Kali ini kita akan membahas tentang rumus mmol. Sebelum itu, mari kita pahami terlebih dahulu tentang konsep mmol. Mmol adalah singkatan dari millimole, yaitu satuan konsentrasi zat dalam kimia. Jadi, mmol.

A comparison of serum sodium concentrations (mmol/ l) of Normal... Download Scientific Diagram
Summary. People can calculate their total cholesterol by adding their levels of high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, and 20% of their.
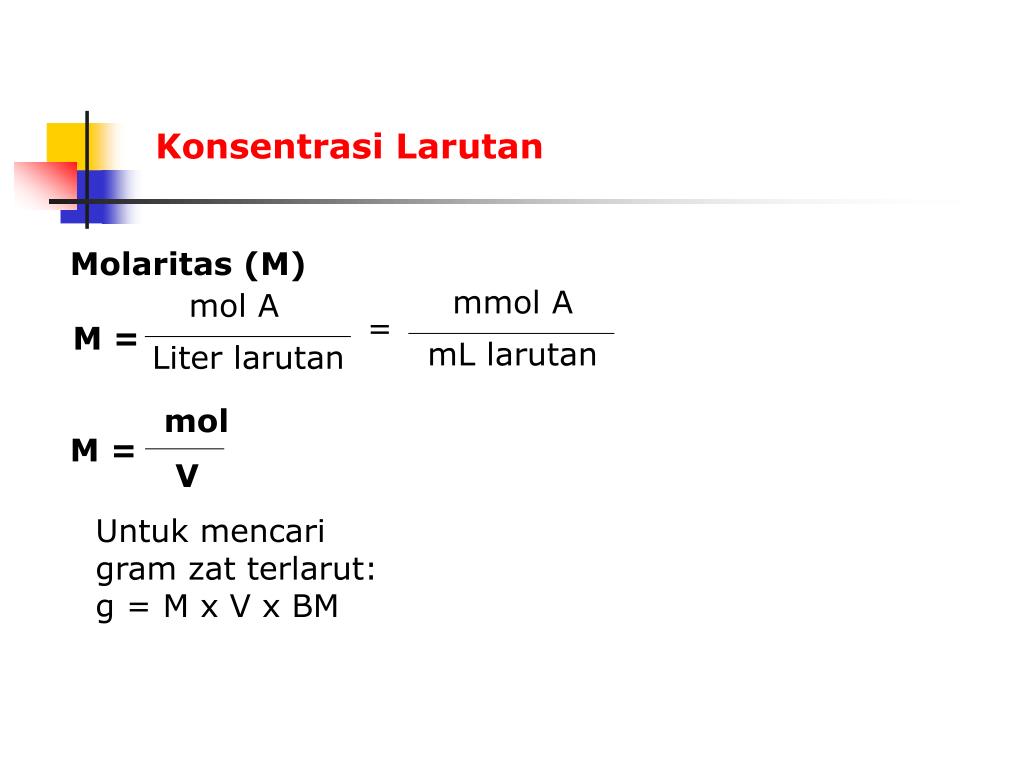
PPT Metode Titrimetri / Volumetri PowerPoint Presentation, free download ID5411283
Tenang, elo juga akan mengetahui rumus molalitas di poin selanjutnya. Tapi sebelum lanjut ke rumus molaritas larutan, gue saranin elo download aplikasi Zenius di gadget masing-masing. Pakai aplikasi Zenius elo bisa nonton penjelasan videonya dengan lebih detail dan mudah dimengerti. Makanya langsung klik banner di bawah ini aja yuk