
SOLVED The Ksp of silver chromate (Ag2CrO4) is 1.12 X1012 (a) What is the solubility of the
Pembahasan. Hasil Kali Kelarutan (Ksp) adalah hasil kali konsentrasi ion-ion dari larutan jenuh garam yang sukar larut dalam air. Setelah itu, masing-masing konsentrasi dipangkatkan dengan koefisien menurut persamaan ionisasinya. Persamaan reaksi ionisasi garam adalah sebagai berikut. Rumus Ksp untuk persamaan reaksi di atas adalah .

Rumus hasil kali kelarutan (KSP) Ag2CrO4 dinyatakan sebag...
Hasil Kali Kelarutan (Ksp) adalah hasil kali konsentrasi ion-ion yang masing-masing dipangkatkan dengan koefisiennya. Ag₂CrO₄ ⇌ 2Ag⁺ + CrO₄²⁻ Rumus Kspnya adalah Ksp = [Ag⁺]² [CrO₄²⁻] Terima kasih telah bertanya di Roboguru, semoga kamu dapat memahami penjelasan diatas. Selamat belajar!

Given the two standard reduction potentials below what is the ksp of ag2cro4 at 25 °c
The equilibrium constant for a dissolution reaction, called the solubility product ( Ksp ), is a measure of the solubility of a compound. Whereas solubility is usually expressed in terms of mass of solute per 100 mL of solvent, Ksp is defined in terms of the molar concentrations of the component ions. In contrast, the ion product ( Q) describes.

The Ksp of Ag2CrO4 is 1.1 × 10^12 at 298 K . The solubility (in mol L^1 ) of Ag2CrO4 in a 0.1
M x A y (s) → x M y+ + y A x-. Karena zat pada tidak mempunyai molaritas, maka tetapan kesetimbangan reaksi diatas hanya melibatkan ion-ionnya saja, dan tetapan kesetimbangannya disebut tetapan hasil kali kelarutan (Ksp).. Ksp = [M y+] x [A x-] y. Hubungan Kelarutan dengan Ksp. Secara umum, hubungan antara kelarutan (s) dengan tetapan hasil kali kelarutan (Ksp) untuk larutan elektrolit A x B.

Rumus hasil kali kelarutan (KSP) Ag2CrO4 dinyatakan sebag...
Rumus Hasil Kali Kelarutan Ksp Ag2Cro4. Untuk menghitung Ksp Ag2Cro4, kita perlu mengetahui persamaan reaksi pembentukannya terlebih dahulu. Reaksi pembentukan Ag2Cro4 dapat dituliskan sebagai berikut: 2AgNO3 + K2CrO4 → Ag2CrO4 + 2KNO3. Dari persamaan reaksi di atas, kita dapat mengetahui bahwa satu mol Ag2CrO4 terbentuk dari satu mol AgNO3.

The Ksp of Ag2CrO4 = 1.2 × 10^11 . What concentration Ag^+ ion in aqueous solution will just
Contoh Soal Kelarutan dan Hasil Kali Kelarutan (KSP) dan Pembahasan Contoh Soal 1: Hitunglah kelarutan Cu(OH) 2 dalam satuan g/L, jika diketahui K sp Cu(OH) 2 = 2,2 × 10 −20. Pembahasan: Contoh Soal 2: Hitunglah kelarutan molar PbI 2 dalam larutan KI 0,1 M. (K sp PbI 2 = 7,1 × 10 −9) Pembahasan: Dalam larutan, KI akan terdisosiasi menjadi.

The Ksp of Ag2CrO4, AgCl, AgBr and Agl are respectively, 1.1 × 10^12, 1.8 × 10^10 , 5.0 × 10
Calculate the solubility of Ag2CrO4 (Ksp = 1.31 x 10-12) a) in pure H2O b) in 0.0100 M AgNO3 c) in 0.0100 MNa2CrO4. 6 comments. Upgrade to add a comment. BP Brendan P. March 12, 2023. the answer provided seems to be option b which is 1.4410-8! but the explanation is, like kinda hard to follow could be clearer! RB.
the ksp of AgCrO4 is 1.1 ×10^ 12 at 298K. the solubility of Ag2CrO4 in a 0.1 M AgNO3 solution is?
The Ksp of Ag2CrO4 is 1.12× 10^-12. What is the solubility (in mol/L) of silver chromate in 1.50 M potassium chromate aq solution? In 1.50 M silver nitrate aq solution? In pure water? I'm assuming I need to write the solubility expression. If I have this right, Ksp=[2Ag]^2[CrO4] which becomes 1.12x10^-12=4s^3. But how to I then incorporate.

The Ksp of Ag2CrO4 , AgCl, AgBr and AgI are respectively, 1.1 × 10^12, 1.8 × 10^10, 5.0 × 10
Ag2CrO4(s) ⇌ 2Ag+(aq) + CrO4^(2-) Rumus Ksp untuk Ag2Cro4 adalah: Ksp = [Ag+]^2[CrO4^2-] Dimana [Ag+] adalah konsentrasi ion perak (silver) dan [CrO4^2-] adalah konsentrasi ion kromat (chromate) dalam larutan jenuh. Cara Mengekspresikan Hasil Kali Kelarutan (Ksp) Ag2CrO4. Setelah kita memiliki nilai Ksp untuk Ag2CrO4, kita perlu.

Jika diketahui Ksp Ag2CrO4=4 x 10^12 , konsentrasi ion
The [Ag+] in a saturated solution of Ag2CrO4 is 4 x 10−4 mol/L. Find Ksp of Ag2CrO4. The K s p of A g 2 C r O 4 is 1.1 × 10 − 12 at 298 K. The solubility (in mol L − 1) of A g 2 C r O 4 in a 0.1 M A g N O 3 solubility is: The ksp of Ag2CrO4 is 1.1 ×10−12 at 298k.
Ag2CrO4 and Ag2C2O4 both are present in a saturated solution in water .Ksp of Ag2CrO4 and
Silver chromate is an inorganic compound with formula Ag 2 CrO 4 which appears as distinctively coloured brown-red crystals. The compound is insoluble and its precipitation is indicative of the reaction between soluble chromate and silver precursor salts (commonly potassium/sodium chromate with silver nitrate). This reaction is important for two uses in the laboratory: in analytical chemistry.

The Ksp of Ag2CrO4 , AgCl, AgBr and AgI are respectively, 1.1 × 10^12, 1.8 × 10^10, 5.0 × 10
Question: Which is the correct Ksp expression for Ag2CrO4 (s) dissolving in water? Ksp = [Ag*12(CrO42-1 Ksp = (Ag*12[Cr3+][02-14 Ksp = [A8+][Cro_2-12 Ksp - [Ag*][Cro42-1 . Show transcribed image text. There are 2 steps to solve this one. Who are the experts?

The Ksp for a sparingly soluble Ag2Cro4, is4 * 1012. The molar solubility of the salt is(1) 2.0
Kelarutan dan Hasil Kali Kelarutan - Kimia Kelas 11. Ksp, larutan berada pada kondisi lewat jenuh (ada endapan). Untuk meningkatkan pemahaman Quipperian tentang materi kelarutan dan hasil kali kelarutan, simak contoh soal berikut. Contoh soal 2 Kelarutan Ag2CrO4 dalam air adalah 10-4 M. Tentukan kelarutan Ag2CrO4 dalam larutan K2CrO4 0,01 M!
Jika Ksp Ag2CrO4 = 4 x10^12, kelarutan Ag2CrO4 da...
Homework Statement I have to calculate the KSP of Ag2CRo4. I did this lab several days ago and I'm completely stumped. If anyone could help me out here, at the least guide me in the right direction so I can get this done as I am getting very frustrated :P Here is the data I have derived thus far. 2.5x10-5 = concentration of AgNO 3 1.5x10-5 = concentration of K 2 CrO 4 Using a concentration.

The Ksp of Ag2CrO4, AgCl, AgBr AgI are respectively, 1.1× 10 12, 1.8 × 10 10, 5.0 × 10 13, 8.3 ×
Jika kelarutan Ag2CrO4 dalam air adalah 5mol/Liter , tentukan hasil kali kelarutan Ag2CrO4 ! Jawab : Ag2CrO4 ⇔ 2Ag+(aq) + CrO4-2(aq) s 2s s Ksp Ag2CrO4 = […
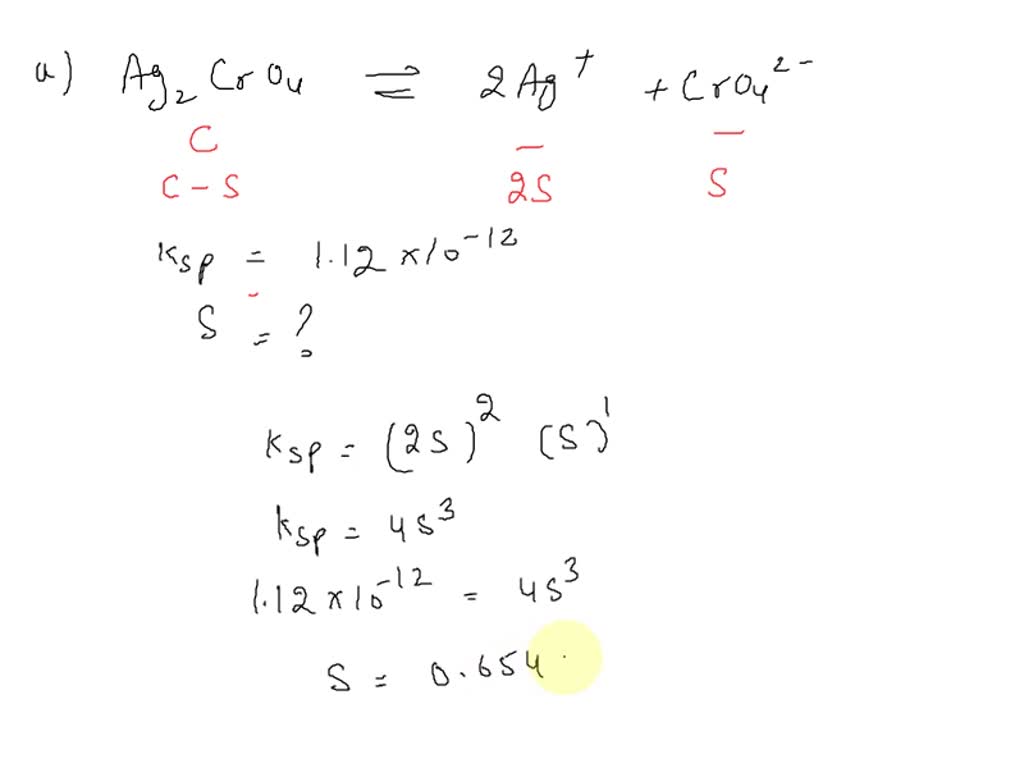
SOLVED Calculate the molar solubility of Ag2CrO4 (Ksp = 1.12×10^12) in (a) Pure water (b) In
Goal. To accurately measure the solubility product constant of silver chromate. Ag2CrO4(s) ( 2 Ag+(aq) + CrO42-(aq)(Reaction One) Introduction. The solubility of a weak ionic electrolyte is measured by a specific type of equilibrium constant called the solubility product constant, Ksp. For a generic binary salt, MaXb, an equilibrium exists.