
Molar Solubility and Ksp K vs Q Comparison YouTube
The solubility product constant, Ksp K s p , is the equilibrium constant for a solid substance dissolving in an aqueous solution. It represents the level at which a solute dissolves in solution. The more soluble a substance is, the higher the Ksp K s p value it has. Consider the general dissolution reaction below (in aqueous solutions):

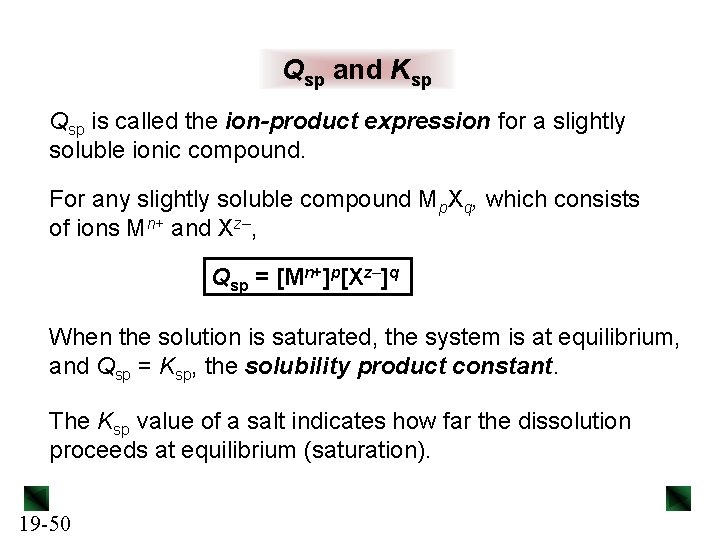
Solubility Equilibria Ksp and Qsp. The dissociation equation is written as a reversible reaction
The solubility product constant Ksp has only one value for a given salt at a specific temperature. That temperature is usually 25 degrees Celsius. And Ksp indicates how much of that salt will dissolve. For example, at 25 degrees Celsius, the Ksp value for barium sulfate is 1.1 times 10 to the negative 10th.

Kelarutan Massa Endapan (Ksp dan Qsp) part 3 YouTube
Therefore the reaction quotient Qsp is equal to the Ksp value for lead II chloride, which means the system is at equilibrium. Adding chloride anion increases the value for Qsp. So now Qsp is greater than Ksp and the system is not at equilibrium. In order to decrease the value for Q, the system needs to move to the left.
PPT Solubility Products, K sp, and Ion Products Q sp PowerPoint Presentation ID4830937
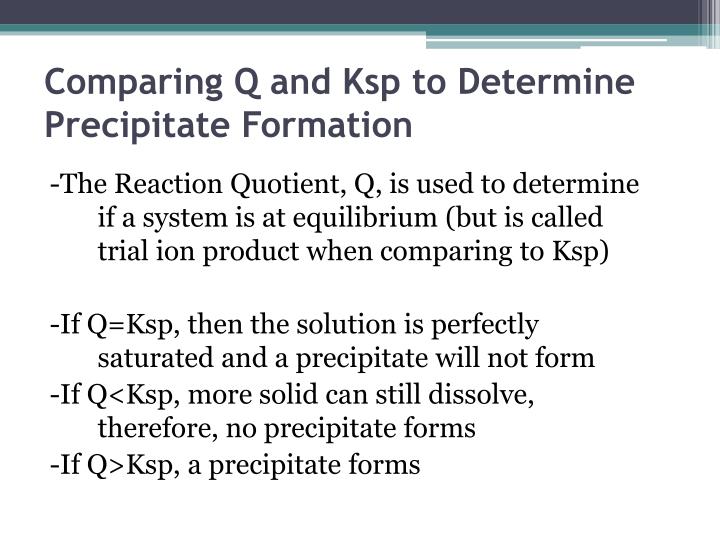
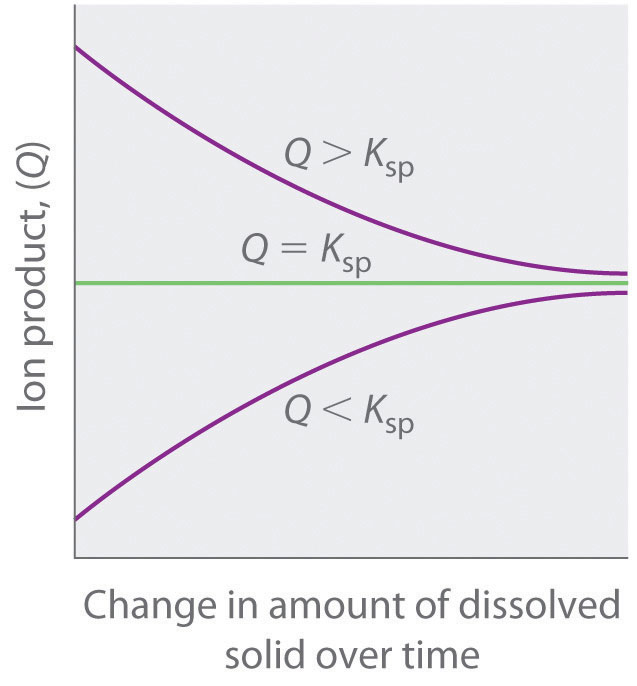
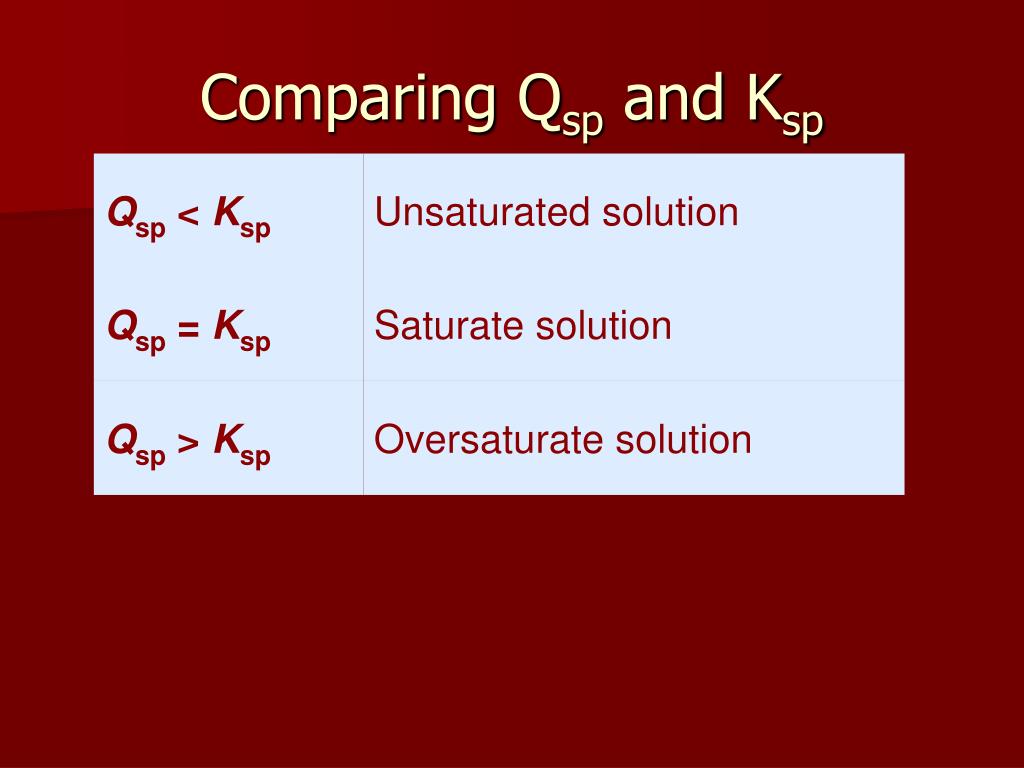
The solubility product quotient (Qsp) has the same equation as Ksp but uses concentrations at a given point in time (typically when the solvation is not equilibrium). Comparing Qsp and Ksp helps determine if a solution is unsaturated (Qsp < Ksp), supersaturated (Qsp > Ksp), or saturated and in equilibrium (Qsp = Ksp).

Comparing Qsp and Ksp to Determine Whether a Precipitate Will Form 001 YouTube
Figure 16.3.1: Above is a diagram of the formation of a precipitate in solution. (Public Domain; ZabMilenko) The use of solubility rules require an understanding of the way that ions react. Most precipitation reactions are single replacement reactions or double replacement reactions.
5.6Qsp and Ksp Science, Chemistry, Chemicalreactions ShowMe
First, write out the Ksp expression, then substitute in concentrations and solve for Ksp: CaF 2 ( s) ↽ − − ⇀ Ca 2 + ( aq) + 2 F − ( aq) A saturated solution is a solution at equilibrium with the solid. Thus: K sp = [ Ca 2 +] [ F −] 2 = ( 2.1 × 10 − 4) ( 4.2 × 10 − 4) 2 = 3.7 × 10 − 11. As with other equilibrium constants.
PPT Ksp The Solubility Product Constant PowerPoint Presentation ID3780287
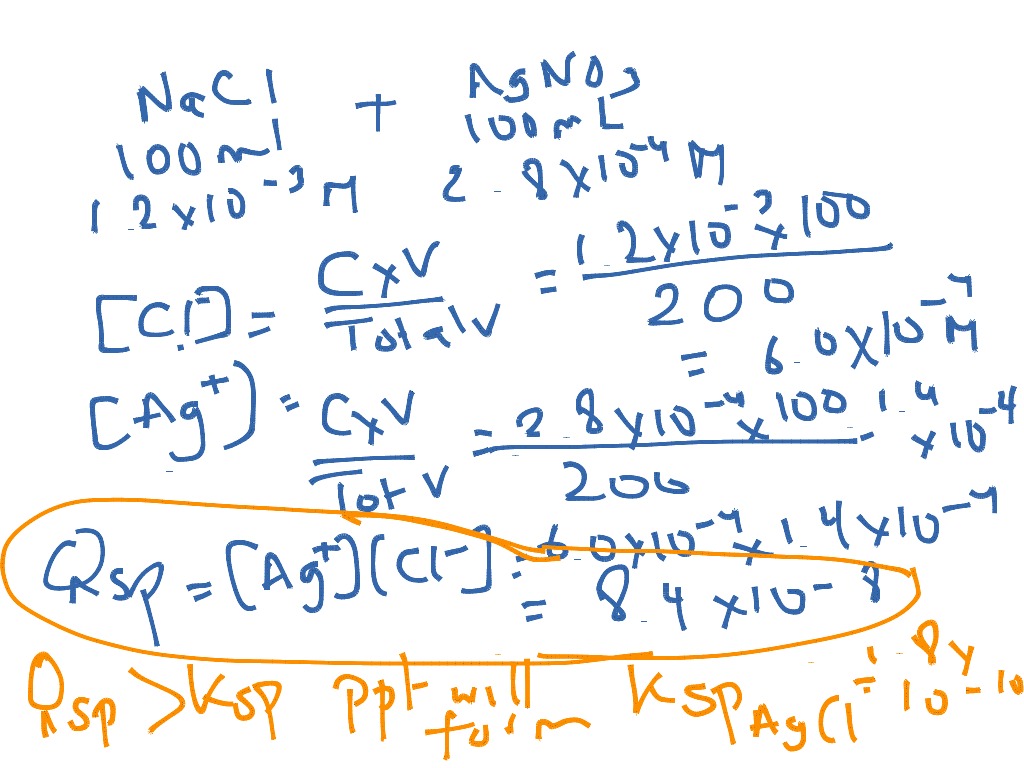
And Ksp = [M n+][X−]n. And likewise we so define Q = [M n+][X−]n. If Q = Ksp, then equilibrium has been reached, and no MACROSCOPIC change will occur. If Q < Ksp, then any precipitate will go up into solution. At Q > Ksp, then precipitation will occur. Answer link. Well, K_"sp" is an actual equilibrium constant, that is experimentally.
Lecture Power Point Chemistry The Molecular Nature of
We can use the reaction quotient to predict whether a precipitate will form when two solutions containing dissolved ionic compounds are mixed. If Q is less t.

Solubility, Precipitation, Ksp, Qsp, Common Ion effect YouTube
QSD/QSP training may be completed before you obtain your underlying certification, but you will not be granted your QSP or QSD certificate until you have an underlying certification. When possible, it is advantageous to complete the underlying certificate prior to the QSD/QSP training. Holistically, both the underlying certificate and the.
Solubility and Complexation Equilibriums
🎯 Want to ace chemistry? Access the best chemistry resource at http://www.conquerchemistry.com/masterclass📗 Need help with chemistry? Download 12 Secrets t.

Qsp Ksp Studyhelp
We look at how to calculate Qsp, and compare this to Ksp in order to see if a solution will precipitate out or not!

PPT Solubility Equilibrium PowerPoint Presentation, free download ID2602304
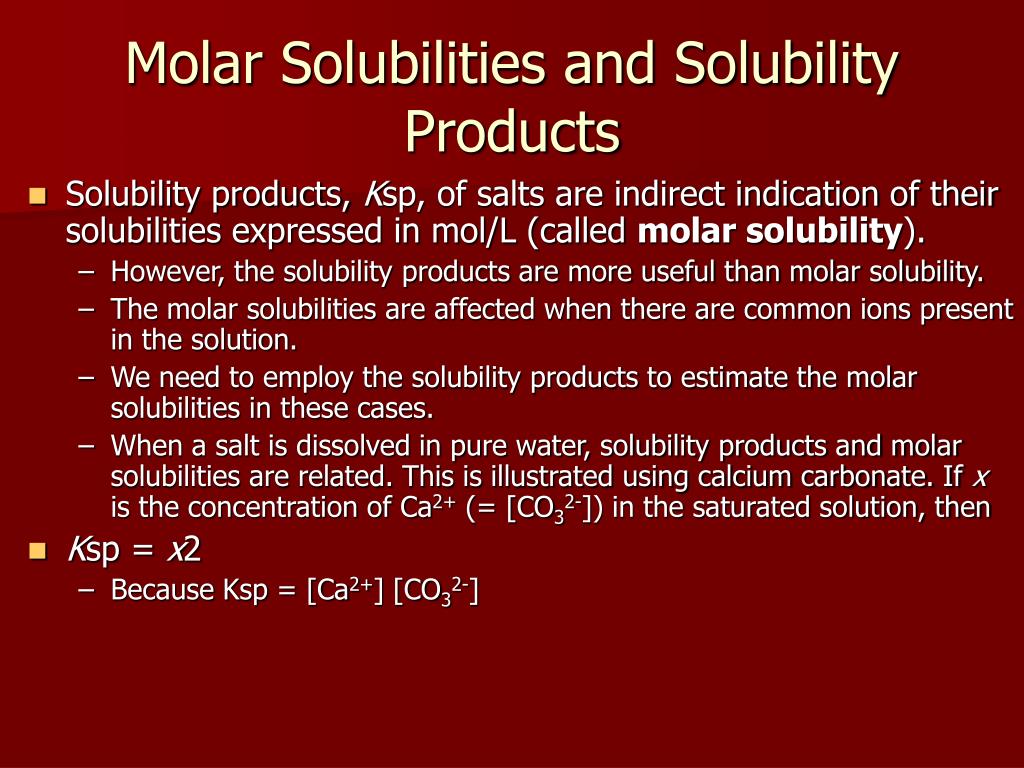
When discussing the molar solubility and solubility product constant (K sp), we mentioned that the values pertain to saturated solutions of the given compound.. For example, the molar solubility of BaOS 4 is 3.87 x 10-5 mol/L. Therefore, it starts precipitating only once the concentration goes higher than 3.87 x 10-5 mol/L which means below this concentration, it is dissociated to ions.

Ksp, Qsp, Solubility Constant, Common Ion Effect Part 2 Grade 12 Chemistry Power Point WITH
At 25 degrees Celsius, the KSP value for lead two sulfate is equal to 6.3 times 10 to the negative seventh. QSP at this moment in time is 5.1 times 10 to the negative six. Therefore QSP is greater than KSP. Since QSP is greater than KSP, we've exceeded the limit of what can dissolve and therefore the solution is oversaturated.

Week 10 12. Precipitation calculation using Qsp and Ksp YouTube
5 min read. The main difference between Ksp and Qsp is that Ksp is a constant value that represents the equilibrium condition for the dissolution of a salt, while Qsp is a variable that represents the current ion concentrations in a solution at any given moment. Both Ksp (solubility product constant) and Qsp (reaction quotient for solubility.
PPT Solubility Products, K sp, and Ion Products Q sp PowerPoint Presentation ID4830937
Slide 0. CH302 Unit 3 Day 1 KSP, PRECIPITATION REACTIONS, INTRO TO KINETICS. K Qs and Saturation - Definitions Solubility Product (KS ): KS is a constant that represents the mass action expression at specific t/ a gleen salt. equilibrium This is the K of the salt dissolution reaction. in KS p calculations. This is your best measurement OF the.

How to Determine if Precipitate will Form or Not Examples, Practice Problems, Qsp Ksp, Step by
Finally, plugging into Ksp and solving, we find: x^2 = 3.36 x 10^(-9) ⇒ x = 3.3*10^(-14).. Relating Qsp and Ksp. Like regular equilibrium, we can also relate Ksp to the reaction quotient to see if a precipitation reaction will produce more or less precipitate to adjust to equilibrium. Like before, if Q > K, the reaction will produce more reactant.