Ondansetron 2mg/mL SDV 2mL/Vial McGuff Medical Products
Buy Ondansetron hydrochloride USP compendial standard (CAS 103639-04-9) to determine strength, quality, purity and identity in your USP-NF monograph tests and assays.

2 mg Ondansetron Hydrochloride Injection, Rs 11.4 /strip Estrellas Life Sciences Private Limited
For IV infusion, dilute ondansetron hydrochloride injection in 50 mL of 5% dextrose injection or 0.9% sodium chloride injection. For IV injection, no dilution required. Rate of Administration. IV infusion: Infuse over 15 minutes. IV injection: Inject over a period of ≥30 seconds, preferably over 2-5 minutes. IM Administration

Pill Identification Images of Ondansetron Hydrochloride Size, Shape, Imprints and Color
To prevent nausea and vomiting associated with cancer chemotherapy the recommended adult oral dosage of ondansetron (ZOFRAN®) is a single 24-mg tablet administered 30 minutes before the start of single-day highly emetogenic chemotherapy; for moderately emetogenic cancer chemotherapy the recommended adult oral dosage is one 8-mg ondansetron (ZOFRAN®) tablet given twice a day (every 12 hours.

Ondansetron Hydrochloride Injection at Best Price in India
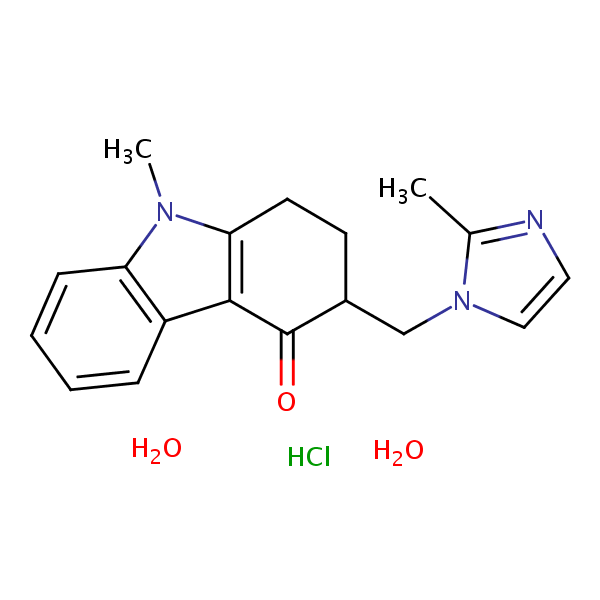
The methyl substituted imidazole ring in the title compound, 2-methyl-1-(9-methyl-4-oxo-2,3,4,9-tetrahydro-1H-carbazol-3-yl) imidazol-3-ium chloride dihydrate, C18H20N3O+.Cl-.2H2O, is approximately perpendicular to the carbazole plane [dihedral angle 87.0(1) degrees]. The water molecules are involve.
Ondansetron hydrochloride dihydrate SIELC
jaundice (yellowing of the skin or eyes); blurred vision or temporary vision loss (lasting from only a few minutes to several hours); high levels of serotonin in the body--agitation, hallucinations, fever, fast heart rate, overactive reflexes, nausea, vomiting, diarrhea, loss of coordination, fainting. Common ondansetron side effects may.

Ondansetron HCL Injection (2mg/ml) 20ml Bottle 1Family 1Health Pharmacy
Find patient medical information for ondansetron HCl oral on WebMD including its uses, side effects and safety, interactions, pictures, warnings and user ratings.

ONDANSETRON HCL 2H2O YARINDO 4 MG TABLET Kegunaan, Efek Samping, Dosis dan Aturan Pakai
Ondansetron is one of the medications most commonly used for the empiric treatment of nausea and vomiting. Ondansetron has excellent utility as an antiemetic drug, and it is effective against nausea and vomiting of various etiologies. Common uses of ondansetron include the prevention of chemotherapy-induced and radiation-induced nausea and vomiting, the prevention of postoperative nausea and.

Ondansetron Hydrochloride Oral Solution, 2 Mg, Packaging Size 30 Ml, Rs 37 /bottle ID
ONDANSETRON SAFETY. There is disagreement in the literature regarding the teratogenic risk of ondansetron use during pregnancy, particularly with regard to cardiac defects and cleft palates. The current dispute arose largely because of the publication of two large, retrospective studies 9, 10 and one abstract, which reported results that.
Ondansetron Hydrochloride Injection Manufacturer and Supplier in India
C18H20ClN3O.2H2O; GR 38032 HCl; Ondansetron hydrochloride, 98%; CHEMBL1201111; Ondansetron hydrochloride- Bio-X; MKBLHFILKIKSQM-UHFFFAOYSA-N; DTXSID701027913;. Ondansetron hydrochloride is also being studied in the treatment of other conditions. NCI Cancer Drugs. 6.6 Clinical Trials. 6.6.1 ClinicalTrials.gov.

ONDANSETRON HYDROCHLORIDE USP DIHYDRATE PCCA
Name. Ondansetron hydrochloride dihydrate. Drug Entry. Ondansetron. A competitive serotonin type 3 receptor antagonist. It is effective in the treatment of nausea and vomiting caused by cytotoxic chemotherapy drugs, including cisplatin, and has reported anxiolytic and neuroleptic properties. Having been developed in the 1980s by GlaxoSmithKline.

Daily Medication Pearl Ondansetron Hydrochloride (Zofran)
Ondansetron is a white to off-white powder that is sparingly soluble in water. Each 1 mL of the preservative-free aqueous solution in the 2-mL single dose vial contains 2 mg of ondansetron as the hydrochloride; 9 mg of sodium chloride; and 0.5 mg of citric acid monohydrate and 0.25 mg of sodium citrate dihydrate as buffers in water for injection.

Ondansetron Hydrochloride Oral Solution IP, 2mg, Rs 12.80 /pack ID 22580267591
Ondansetron hydrochloride is a member of carbazoles. ChEBI. Ondansetron Hydrochloride is the hydrochloride salt of the racemic form of ondansetron, a carbazole derivative and a selective, competitive serotonin 5-hydroxytryptamine type 3 (5-HT3) receptor antagonist with antiemetic activity.
Ondansetron HCL Injection (2mg/ml) 20ml Bottle 1Family 1Health Pharmacy
The molecular formula is C18H19N3O•HCl•2H2O,. Ondansetron tablets, USP for oral administration contain ondansetron hydrochloride USP (dihydrate) equivalent to 4 mg or 8 mg or 24 mg of ondansetron. Each film-coated tablet also contains the inactive ingredients anhydrous lactose, microcrystalline cellulose, pregelatinized starch (maize.
Ondansetron 2mg/mL SDV 2mL/Vial McGuff Medical Products
The molecular formula is C18H19N3O•HCl•2H2O,. Ondansetron tablets, USP for oral administration contain ondansetron hydrochloride USP (dihydrate) equivalent to 4 mg or 8 mg or 24 mg of ondansetron. Each film-coated tablet also contains the inactive ingredients anhydrous lactose, microcrystalline cellulose, pregelatinized starch (maize.

Ondansetron hydrochloride United States Pharmacopeia (USP) Reference Standard Ondansetron
Ondansetron Tablets are a product of USP, a scientific organization that sets standards for the quality and purity of medicines. Ondansetron is a medication used to prevent nausea and vomiting caused by chemotherapy, radiation, or surgery. Learn more about the specifications, ingredients, and uses of Ondansetron Tablets from USP.

Pill Identifier Ondansetron Hydrochloride NDC 55111154
1.5 Appearance. Ondansetron hydrochloride is obtained as a white or off-white powder.. 1.6 Uses and Applications. Ondansetron hydrochloride is the soluble form of ondansetron, a tetrahydrocarbazolone derivative with an imidazolylmethyyl group. It is the first of a class of selective serotonin 5-HT3 receptor antagonists indicated for the prevention of nausea and vomiting associated with initial.