
Represente A Estrutura De Lewis Para O Nh3 Várias Estruturas
Ammonia (NH 3) is a commonly tested Lewis structure due to it's widespread use in agriculture as a fertilizer. It also is a good example of a molecule with a trigonal prymidal molecular geometry. There are 8 valence electrons available for the Lewis structure for NH 3. Video: Drawing the Lewis Structure for NH3 It is helpful if you:

Gambarkan Struktur Lewis Nh3
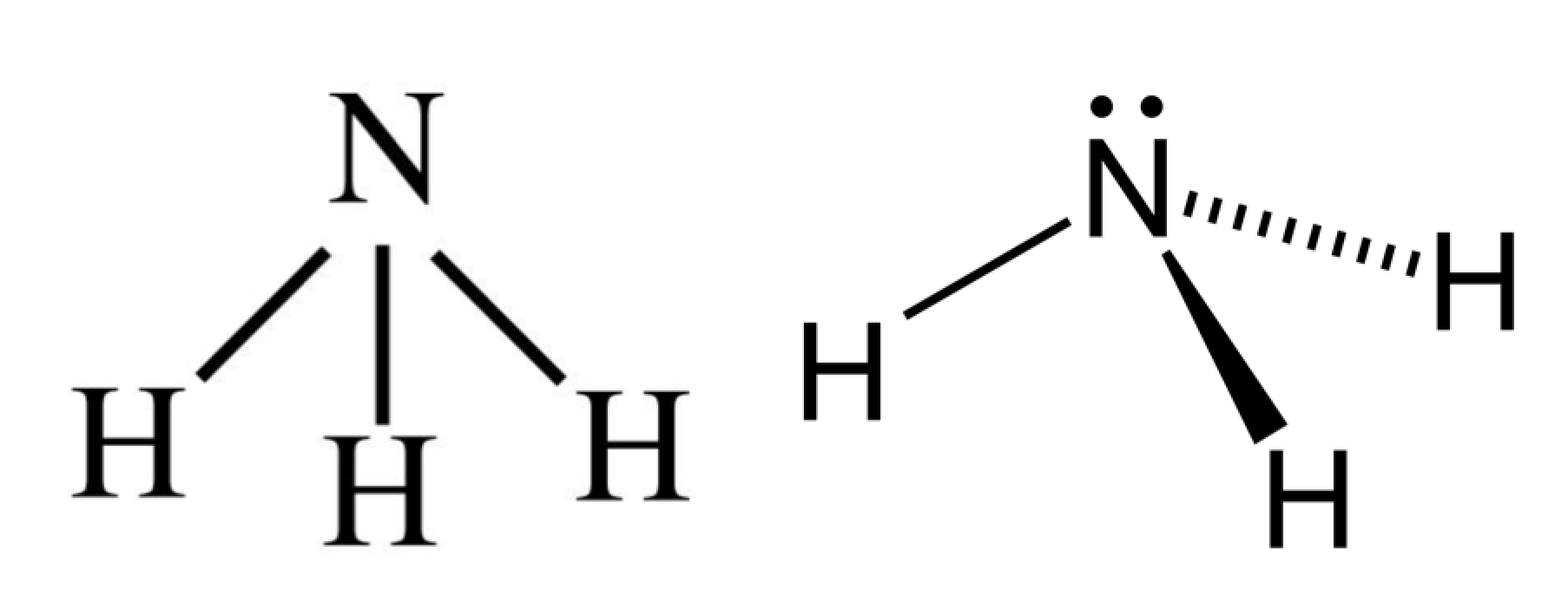
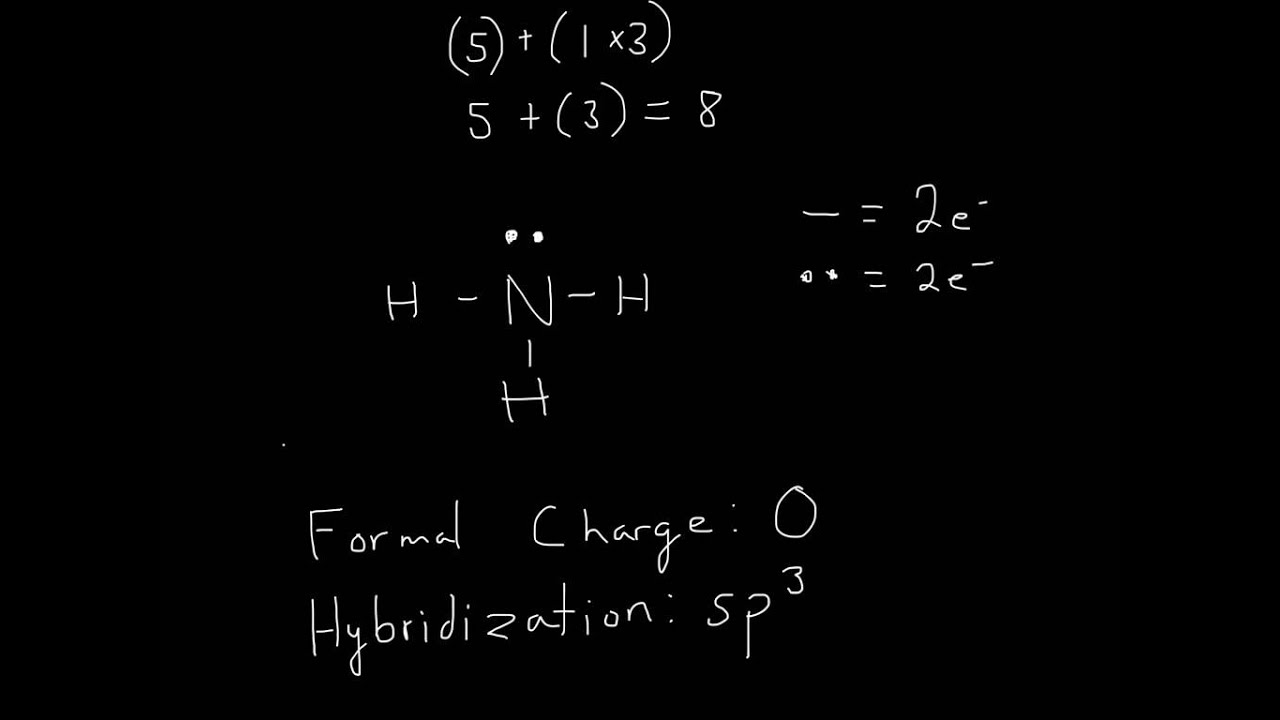
In the Lewis structure for NH 3 there are a total of 8 valence electrons. Three pairs will be used in the chemical bonds between the N and H and one pair of electrons will be unbonded. Transcript: OK, this is Dr. B. We're going to do the Lewis structure for NH3: ammonia or Nitrogen trihydride. On the periodic table, Nitrogen is in group 5 or 15.

Lewis Structure of NH3 (Ammonia) YouTube
NH3 Lewis Structure: NH3 (Ammonia) has a trigonal pyramidal structure: central N atom with 5 valence electrons forms 3 N-H single bonds, using 3H atoms (1 electron each), and 1 lone pair on N. Bond angle: 107.8°, due to lone pair repulsion. Valence shell electron pair repulsion (VSEPR) theory explains shape; electron geometry: tetrahedral. ad
Nh3 Molecule Structure
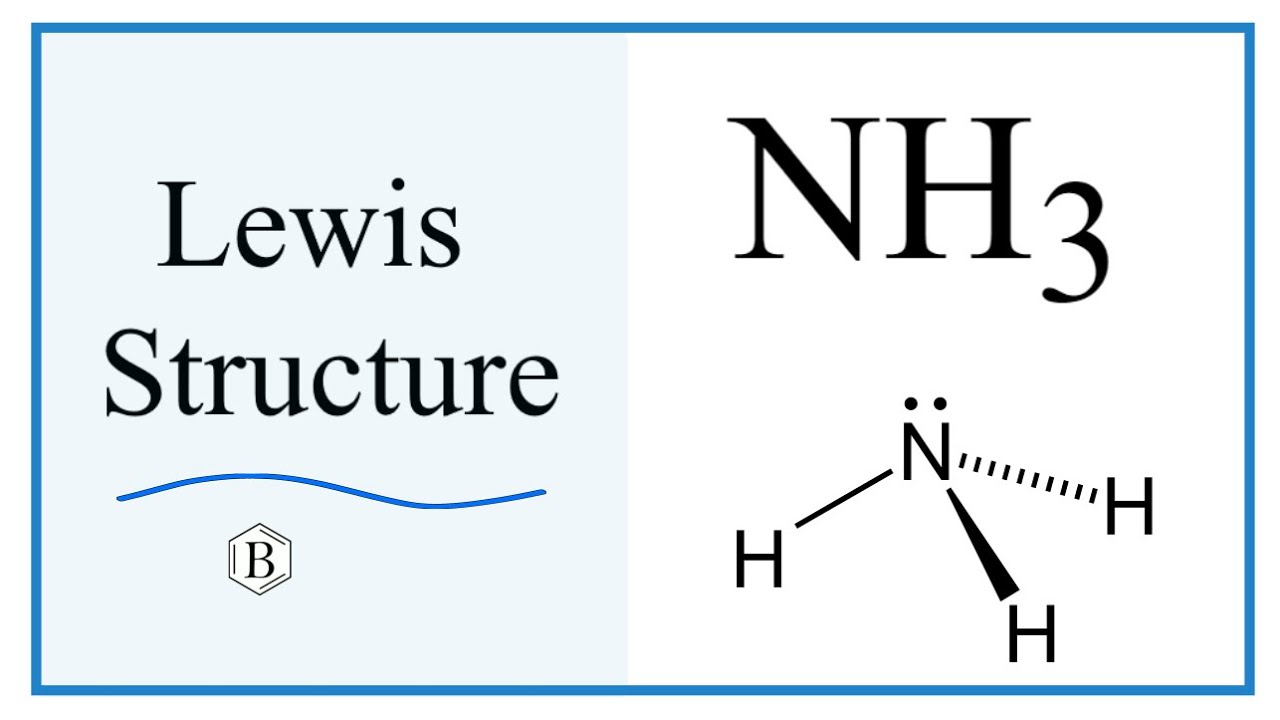
Answer: The NH3 Lewis structure represents the arrangement of atoms and bonding electrons in ammonia. It consists of one nitrogen atom (N) bonded to three hydrogen atoms (H). The central nitrogen atom has a lone pair of electrons, represented by two dots, and forms three covalent bonds with the hydrogen atoms.

NH3 Molecular Geometry Science Education and Tutorials
Pengertian Struktur Lewis Struktur Lewis adalah penggambaran distribusi elektron dalam suatu struktur molekul dengan menggunakan tanda elektron. Tanda elektron yang digunakan, biasanya berupa tanda titik (.) dan tanda silang (x), sehingga teori Lewis ini sering juga disebut sebagai teori dot-cross Lewis.

Nh3 Estrutura De Lewis
Steps. To properly draw the NH 3 Lewis structure, follow these steps: #1 Draw a rough sketch of the structure. #2 Next, indicate lone pairs on the atoms. #3 Indicate formal charges on the atoms, if necessary. Let's break down each step in more detail.

Estructura de Lewis NH3, Amoniaco » Quimica Online
Ammonia Lewis structure and more.

Estructura de Lewis NH3, Amoniaco » Quimica Online
Understanding the NH3 Lewis structure is crucial for comprehending the chemical properties and behavior of ammonia. By following the step-by-step guide provided in this article, you can draw the NH3 Lewis structure accurately and gain insights into its molecular bonding.

Gambarkan Struktur Lewis Nh3
Ammonia NH3 Lewis Dot Structure shadowboy220 1.9K subscribers Subscribe Subscribed 49 Share 16K views 11 years ago Chemistry Lewis Dot Structures A video explanation of how to draw the Lewis.

NH3 Lewis Structure How to Draw the Dot Structure for NH3 YouTube
NH3 (Ammonia) lewis structure has a Nitrogen atom (N) at the center which is surrounded by three Hydrogen atoms (H). There are 3 single bonds between the Nitrogen atom (N) and each Hydrogen atom (H). There is 1 lone pair on the Nitrogen atom (N).

Lewis Structure NH3 plus dipoles, shape, angles and formal charge YouTube
In the NH3 lewis dot structure, there are Four atoms present, one N and three H atoms. The valence electrons for N are 5 and for three H atoms are 3. So, the total valence electrons are 5+3 = 8. Step 2 - According to the octet rule, the electrons that will be needed for the NH3 lewis dot structure will be 8 + (3*2) = 14.
Lewis Dot Diagram For Nh3 exatin.info
Lewis structure of NH3 (ammonia) contains three single bonds between the Nitrogen (N) atom and each Hydrogen (H) atom. The Nitrogen atom (N) is at the center and it is surrounded by 3 Hydrogen atoms (H). The Nitrogen atom has one lone pair. Let's draw and understand this lewis dot structure step by step.
Struktur Lewis Nh3 Buku Sinta
April 22, 2020 NH3 Lewis Structure, Molecular Geometry, Hybridization, Bond Angle & Shape Ammonia is a colorless compound, used in making fertilizers. It is a stable hydride formed of one nitrogen and three hydrogen atoms. The molecule has a pungent smell. It can form an NH4+ ion by accepting a proton.

How to draw NH3 Lewis Structure? Science Education and Tutorials
Lewis Structure Finder. This widget gets the Lewis structure of chemical compounds. Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.
Lewis Structure of NH3 YouTube
2.8K 493K views 10 years ago NH3 Lewis, Shape, Hybridization, Polarity, and more. A step-by-step explanation of how to draw the NH3 Lewis Dot Structure (Ammonia). For the NH3 structure.

Lewis Dot Structure Of Nh3
To sketch the NH3 Lewis structure by following these instructions: Step-1: NH3 Lewis dot Structure by counting valence electrons on the nitrogen atom. Step-2: Lewis Structure of NH3 for constructing around the central nitrogen atom. Step-3: Lewis dot Structure for NH3 generated from step-1 and step-2.