
Bf3 Chemistry
Highly flammable liquid and vapour. Contains gas under pressure; may explode if heated. Toxic if swallowed. Fatal in contact with skin. Causes severe skin burns and eye damage. Fatal if inhaled. Hazard statements. Precautionary statements: Prevention: - Keep away from heat/sparks/open flames/hot surfaces.
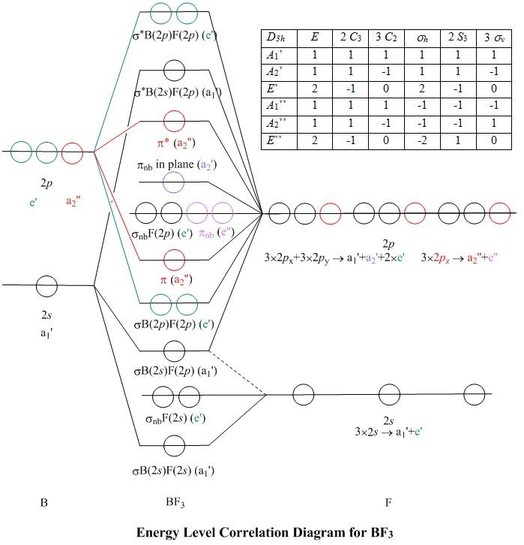
Molecular Orbitals for BF3
Molecular Orbitals for BF3. Jmol models of wavefunctions calculated at the RHF/3-21G* level. To view a model, click in a molecular orbital circle in the energy level correlation diagram shown. Ignore any popup warning and click on the green Continue button which appears. The energy level diagram may be displayed with or without the group theory.
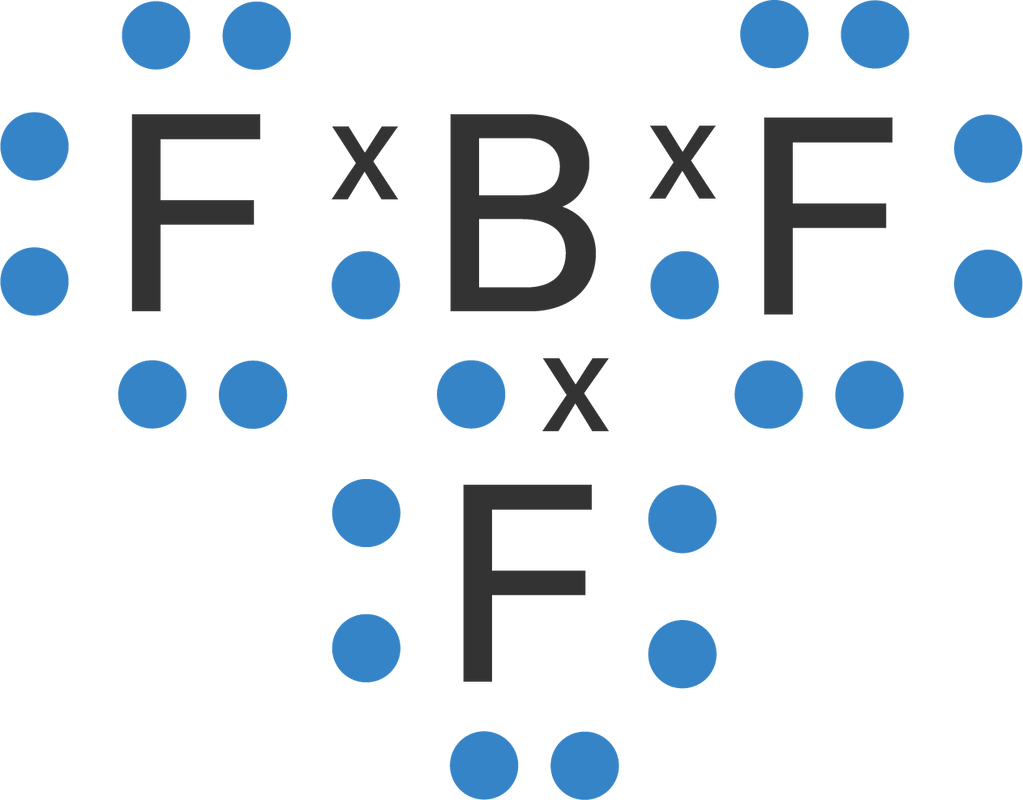
Tuliskan struktur Lewis dan struktur molekul dari
BF3, also known as Boron Trifluoride, is an inorganic chemical compound which is a colorless gas with a pungent smell. Students often get confused regarding the polarity or non-polarity of BF3 (Boron Trifluoride) due to the presence of three Fluorine atoms which have a very high electronegativity value when compared to the Boron atom.

Lewis Dot Structure For Bf3
5.4.6: BF₃. The case of boron trifluoride ( BF3 BF 3) is an example of a molecule with one more layer of complexity than the other examples we have seen in previous sections in this chapter. ( BF3 BF 3) is more complex than previous examples because it is the first case in which there are multiple types of valence orbitals on the pendant atoms.

BF3 Lewis Dot Structure
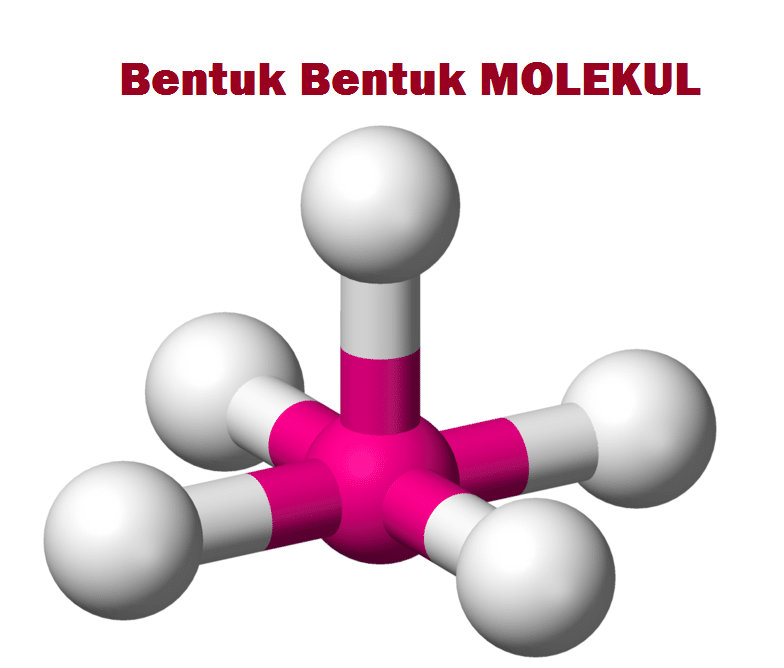
Molecular Geometry of BF3. The geometry of molecule of BF3 is 'Trigonal Planar.'. With the reference of Chemistry, 'Trigonal Planar' is a model with three atoms around one atom in the middle. It's like peripheral atoms all in one plane, as all three of them are similar with the 120° bond angles on each that makes them an equilateral.

Leave a Comment Cancel Reply
BeCl2 Lewis Structure. The electrons present in the outermost shell of an atom are shown in the Lewis structure of any molecule. These electrons will be both bonding as well as non-bonding electrons. The electronic configuration of beryllium is [He] 2s2and chlorine is [Ne] 3s23p5. The number of electrons on the valence shell of Be and Cl is 2.

How to draw BF3 Lewis Structure? 5
Bonding electrons − Antibonding electrons 2 ⋅ number of bonds. which for this diagram gives a value of 43 per bond. This gives a sense of why BFX3 is a strong Lewis acid, since it can accept a pair of electrons into its antibonding LUMO and still leave a compound with a BO of 1 per bond.

Best Overview on BF3 Polar or Nonpolar [1] Science Education and Tutorials
These three hybrid orbitals overlap with fluorine's 2p orbitals. The electron geometry of BF 3 is trigonal planar. The shape is not distorted because there are no lone pairs on the central boron atom. The molecular geometry is the same as the electron geometry. For a trigonal planar structure, the bond angle is 120°. The VSEPR notation is AX 3.

Molecular orbital diagram for BF3 Chemistry Stack Exchange
Tutorial ChemsketchMenggambar geometri molekul atau bentuk molekul BeCl2, BH3, BF3 menggunakan Chemsketch bila dilakukan optimasi lebih dahulu semua seolah t.
Pasangan Gambar Yang Merupakan Molekul Senyawa Adalah denah
Boron trifluoride is the inorganic compound with the formula BF3. This pungent, colourless, and toxic gas forms white fumes in moist air. It is a useful Lewis acid and a versatile building block for other boron compounds.

How to tell bond order of BF3 using molecular orbital theory Chemistry Chemical Bonding and
Berikut gambar struktur Lewis dan bentuk molekul dari BF3: Perbesar. Gambar struktur Lewis dan bentuk molekul dari BF3 (buku Bongkar Pola Soal UNBK SMA/MA IPA) Untuk meminimalkan tolakan, maka ketiga pasangan elektron tersebut masing-maisng akan menempati sudur pada segitiga sama sisi pada bidang datar. Sudut yang terbentuk sebesar 120°.
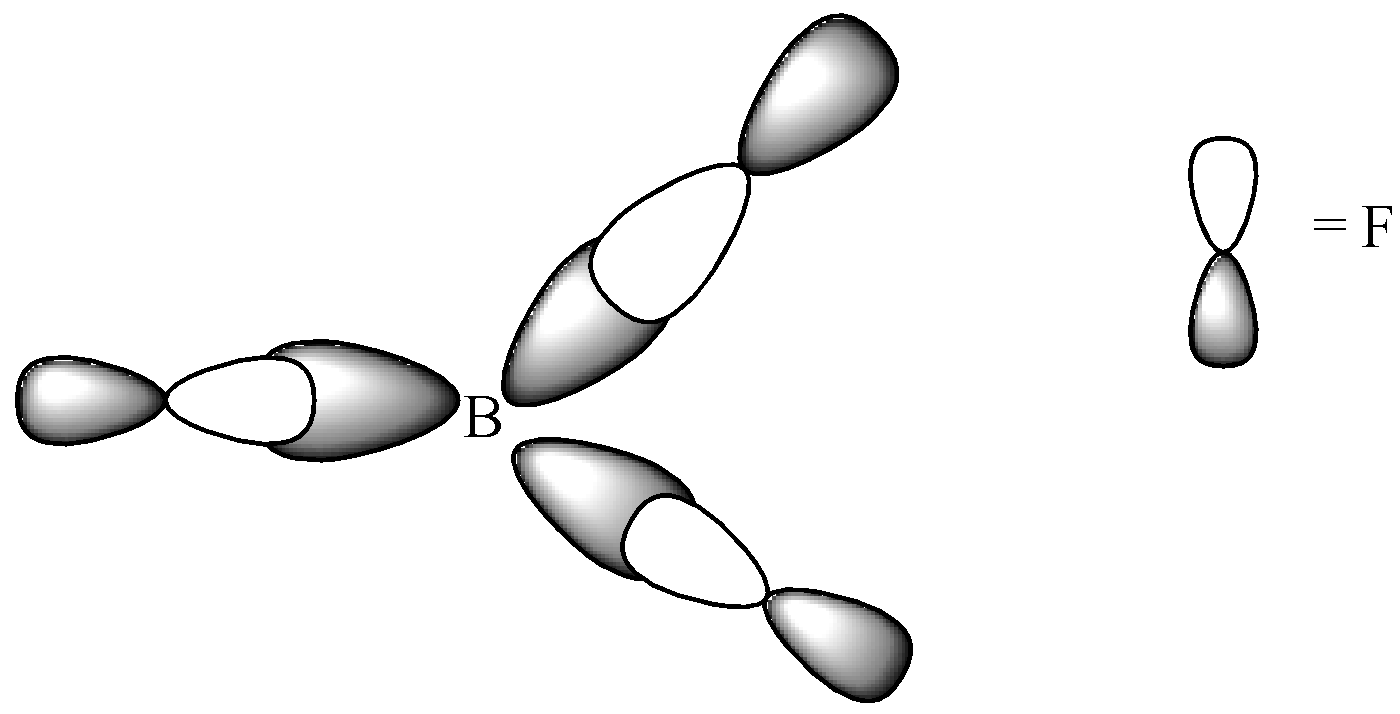
Explain the formation of the B{{F}_{3}} molecule using hybridisation.
BF3 molecular weight. Molar mass of BF3 = 67.8062096 g/mol. This compound is also known as Boron Trifluoride. Convert grams BF3 to moles. or. moles BF3 to grams. Molecular weight calculation: 10.811 + 18.9984032*3. Percent composition by element. Element: Boron Symbol: B Atomic Mass: 10.811 # of Atoms: 1

BF3 Lewis Structure, Molecular Geometry, and Hybridization Techiescientist
Pembahasan Boron (B) memiliki konfigurasi elektron Total elektron valensi 3 elektron PEB =0 PEI = 3 Fluor (F) memiliki konfigurasi elektron Total elektron valensi 7 elektron PEB = 3 PEI = 1 Boron sebagai atom pusat, maka rumus tipe molekulnya adalah AX 3 dengan bentuk molekul trigonal planar Maka, jawaban yang tepat adalah (A)

BF3 Molecular Geometry, Shape and Bond Angles (Boron Trifluoride) YouTube
The complete molecular orbital scheme of BF3 is developed here.
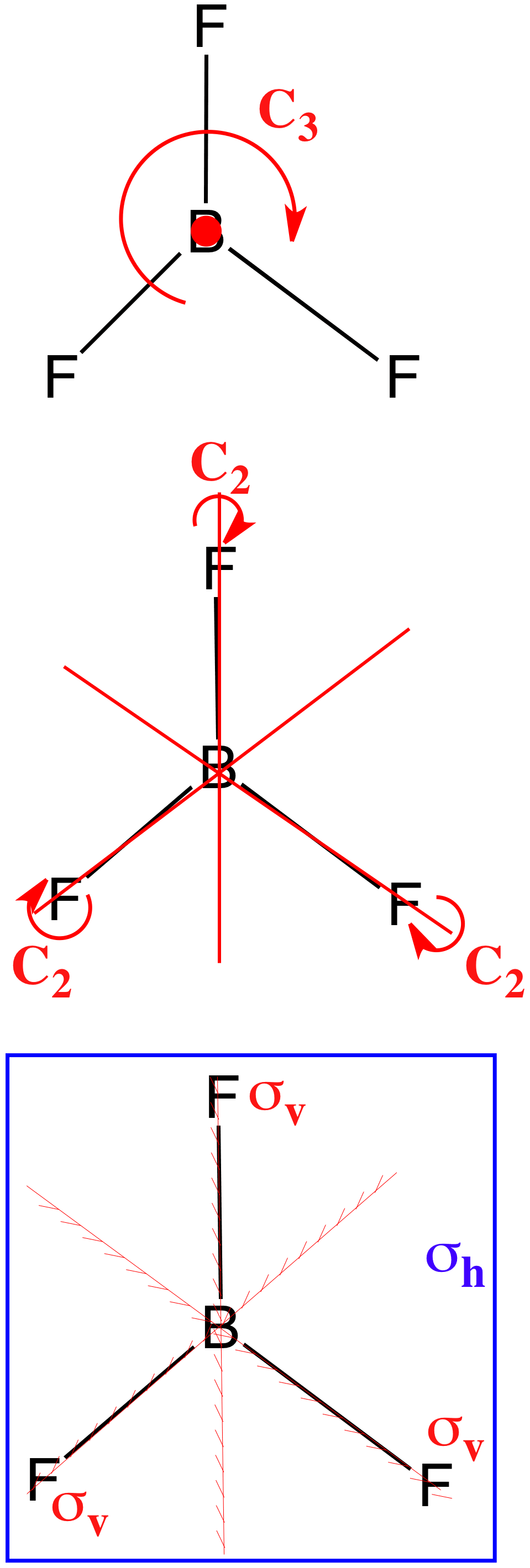
Boron Trifluoride (BF3) D3h
The BF3 molecular geometry is a diagram that illustrates the number of valence electrons and bond electron pairs in the BF3 molecule in a specific geometric manner. The geometry of the BF3 molecule can then be predicted using the Valence Shell Electron Pair Repulsion Theory (VSEPR Theory) and molecular hybridization theory, which states that.

Bentuk Molekul BF3 MateriKimia
Suatu molekul dengan 1 atom pusat yang memiliki 3 PEI dan 0 PEB memiliki bentuk molekul segitiga planar dengan sudut ikatan . Jadi, jawaban yang tepat adalah D. Perdalam pemahamanmu bersama Master Teacher di sesi Live Teaching, GRATIS! 3. 0.0 (0 rating) Iklan. Iklan. Klaim Gold gratis sekarang! Dengan Gold kamu bisa tanya soal ke Forum.