
SNC1P
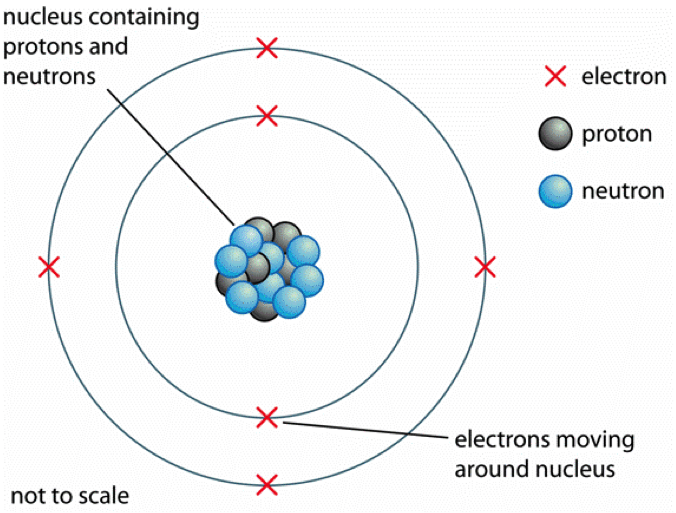
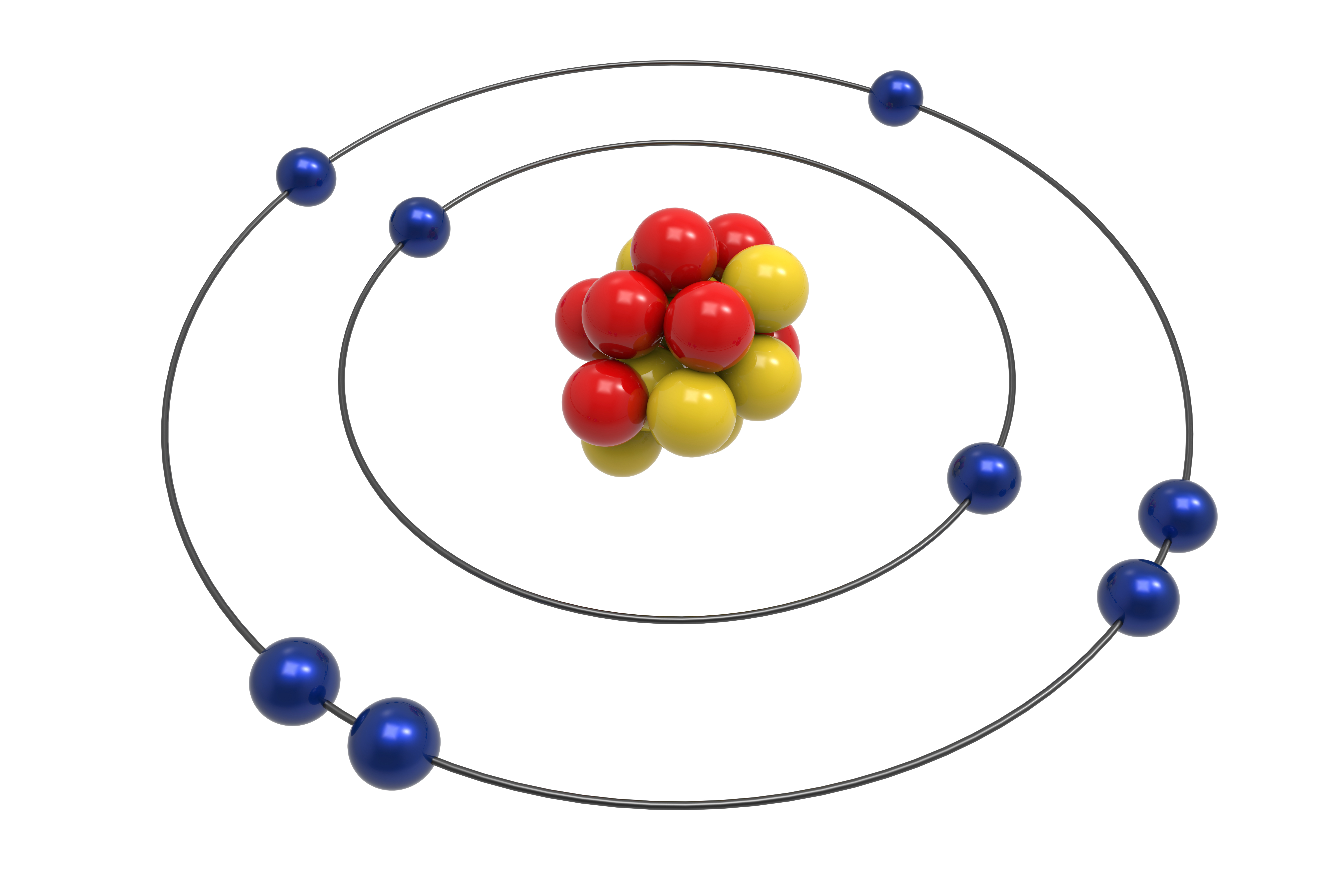
The modern model of the atom. Today, scientists use an atomic model that has a central, positively-charged nucleus that contains: positively-charged protons close proton Subatomic particle with a.
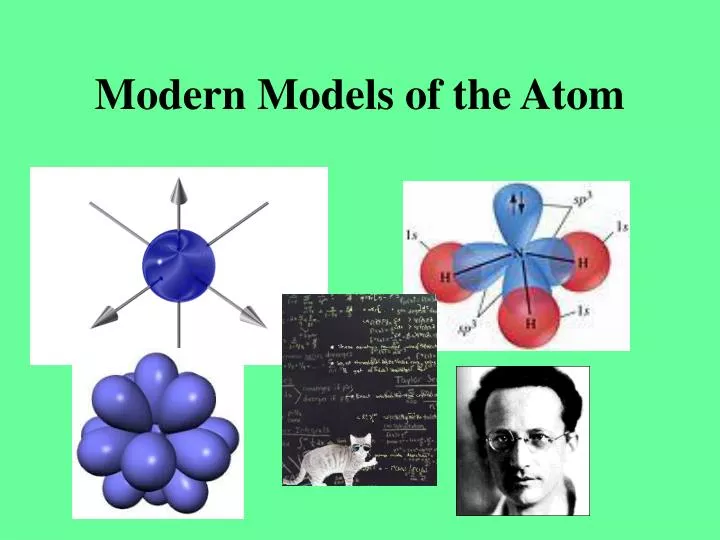
PPT Modern Models of the Atom PowerPoint Presentation, free download ID1259477
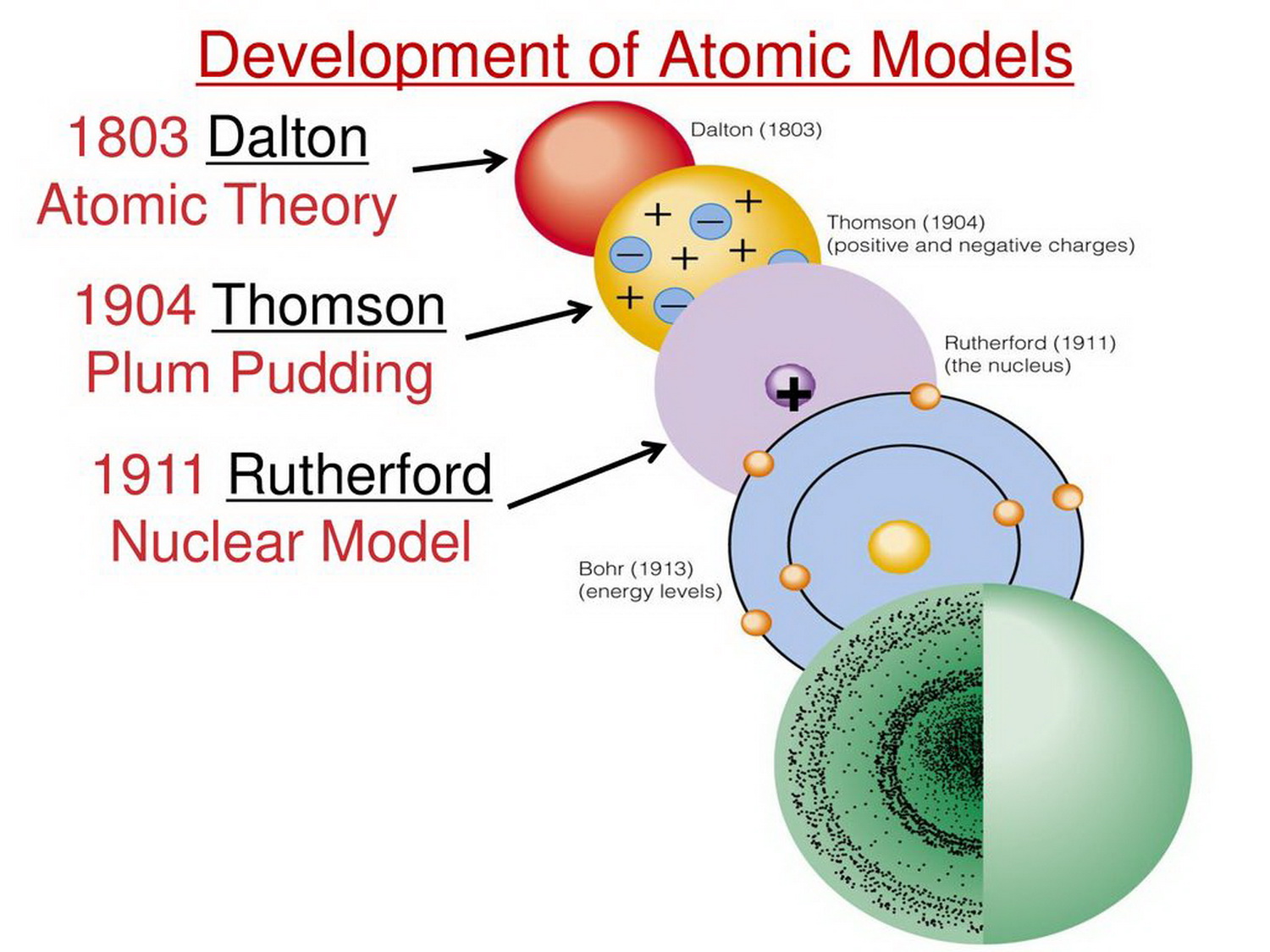
The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. As experiments revealed more about subatomic particles, atomic models evolved from Thomson's "plum pudding model," to Rutherford's nuclear model, then to Niels Bohr's planetary model, and eventually to the currently-accepted quantum-mechanical model.
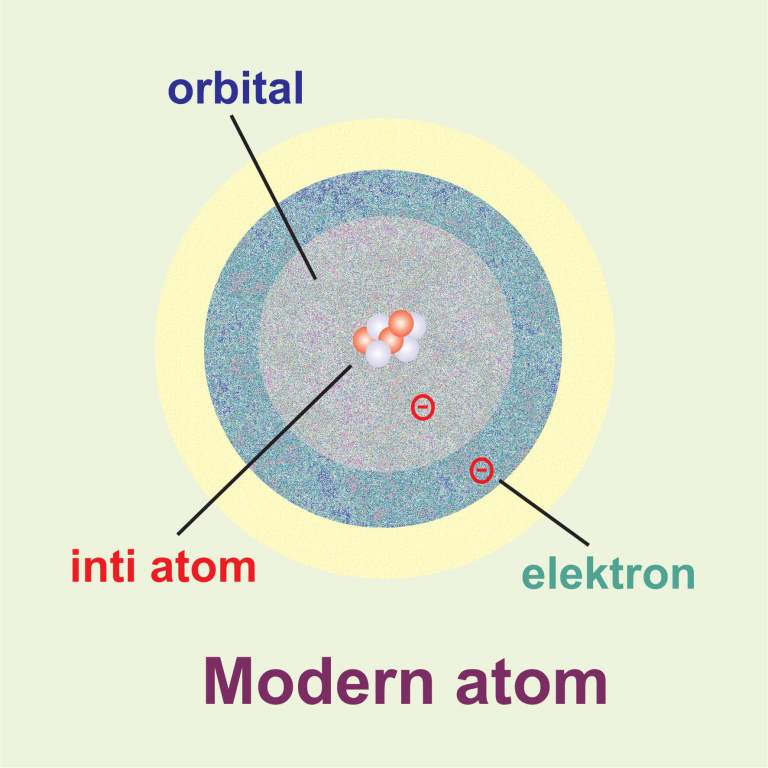
Kelebihan Dan Kelemahan Atom Modern
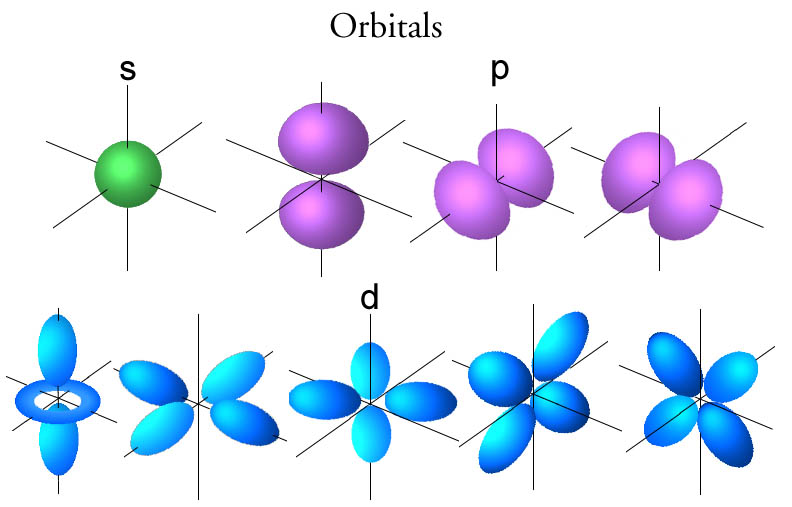
given by the following equation: λ = h m v. Erwin Schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Schrödinger's equation, H ^ ψ = E ψ. . , can be solved to yield a series of wave function ψ. . , each of which is associated with an electron binding energy, E. .

The Modern Atomic Model Can Best Be Described as
A = number of protons + number of neutrons = mass number. Element. Chemical Symbol (X) Atomic Number (Z) Mass Number (A) One or more atoms of the same type. A one or two letter abbreviation assigned to each element. The number of protons in the nucleus of an atom. The type of atom/element is determined by the number of protons.

Belajar 5 Teori Model Atom Dan Penjelasannya, Menarik Untuk Di Simak Ilmusaku
The Bohr model of the atom. The modern atomic model. Resources. The atom is defined as the smallest part of an element that retains the chemical properties of that element. The existence of atoms was first guessed as early as 400 BC, when Greek philosophers debated whether one could divide a substance into infinitely smaller pieces or if.

The Modern Atomic Model
Dalton's ideas proved foundational to modern atomic theory. However, one of his underlying assumptions was later shown to be incorrect. Dalton thought that atoms were the smallest units of matter − tiny, hard spheres that could not be broken down any further. This assumption persisted until experiments in physics showed that the atom was composed of even smaller particles.
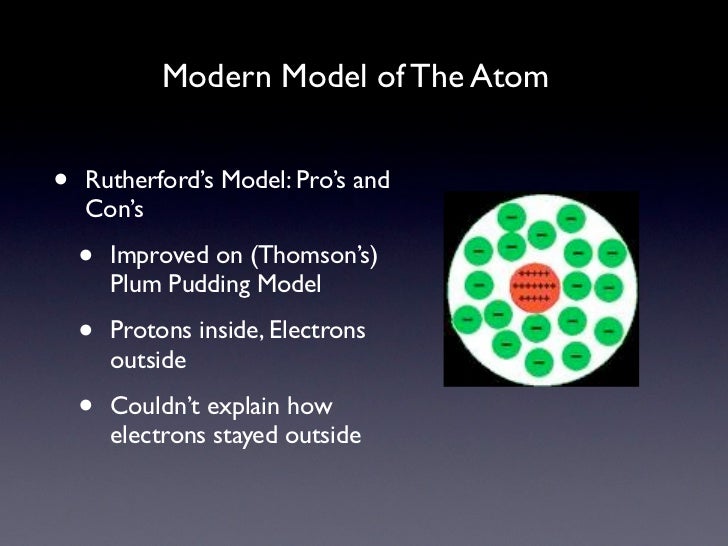
Modern Model of the Atom
Bohr's model and the current model are described in Chapter 6, "The Structure of Atoms." Image used with Permission (CC BY-SA-NC). Rutherford's model of the atom is essentially the same as the modern model, except that it is now known that electrons are not uniformly distributed throughout an atom's volume.
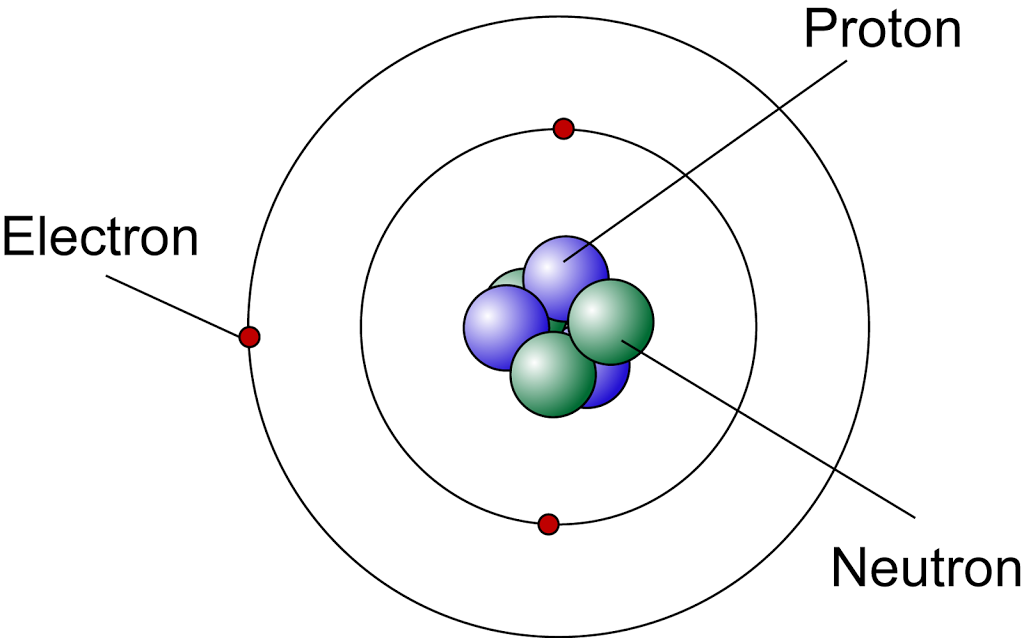
Modern Atomic Model SPM Chemistry
The modern atomic theory, proposed about 1803 by the English chemist John Dalton (Figure 1.5.4 1.5. 4 ), is a fundamental concept that states that all elements are composed of atoms. Previously, an atom was defined as the smallest part of an element that maintains the identity of that element.
/GettyImages-141483984-56a133b65f9b58b7d0bcfdb1.jpg)
Basic Model of the Atom Atomic Theory
The modern atomic model represents atoms containing a nucleus of protons and neutrons and a vague gradient or cloud surrounding it containing the electrons; this is sometimes referred to as the.
Atomic Structure & The Changing Models of Atom
Atomic model, in physics, a model used to describe the structure and makeup of an atom. Atomic models have gone through many changes over time, evolving as necessary to fit experimental data. For a more in-depth discussion of the history of atomic models, see atom: development of atomic theory.
IB Chemistry SL & HL 2.1 Atomic Model
Atom - Dalton, Bohr, Rutherford: English chemist and physicist John Dalton extended Proust's work and converted the atomic philosophy of the Greeks into a scientific theory between 1803 and 1808. His book A New System of Chemical Philosophy (Part I, 1808; Part II, 1810) was the first application of atomic theory to chemistry. It provided a physical picture of how elements combine to form.
Atomic Theory The Modern Atom
From the era of ancient Greek philosophy to modern quantum mechanics, the atomic theory has had multiple updates, each of which was quite revolutionary for its time.. Erwin Schrödinger came up with the quantum mechanical model of an atom. In this model, the electrons do not revolve around the nucleus in circular orbits, but rather as.
Modern Atomic Model
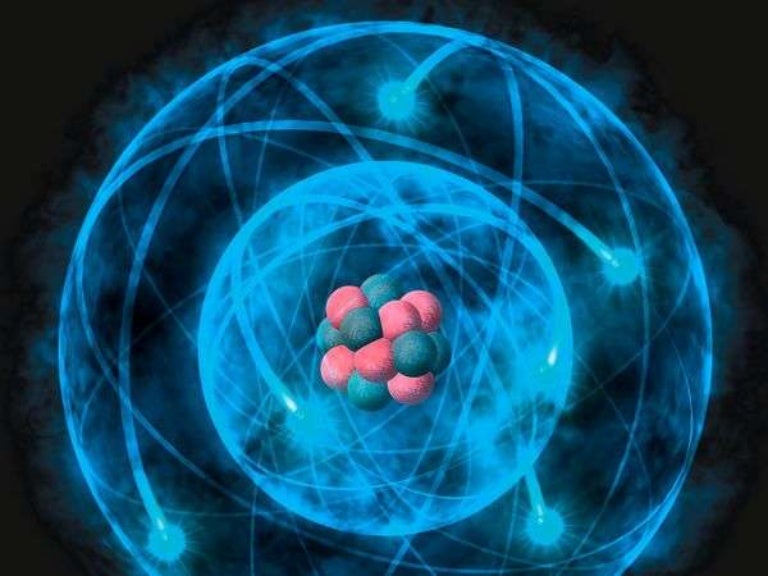
The current theoretical model of the atom involves a dense nucleus surrounded by a probabilistic "cloud" of electrons. Atomic theory is the scientific theory that matter is composed of particles called atoms.The concept that matter is composed of discrete particles is an ancient idea, but gained scientific credence in the 18th and 19th centuries when scientists found it could explain the.
Atomic Models Definition
First published in 1807, many of Dalton's hypotheses about the microscopic features of matter are still valid in modern atomic theory. Here are the postulates of Dalton's atomic theory. Matter is composed of exceedingly small particles called atoms. An atom is the smallest unit of an element that can participate in a chemical change.

Model Model Atom Menurut Para Ahli Seputar Model
In general, the Bohr model encapsulates the modern understanding of the atom. This model is frequently depicted with a central atomic nucleus and oval lines representing electron orbits. The current model of the atom is the "quantum mechanical model" or the "electron cloud model" , which was developed in the 1920s and early 1930s by a.
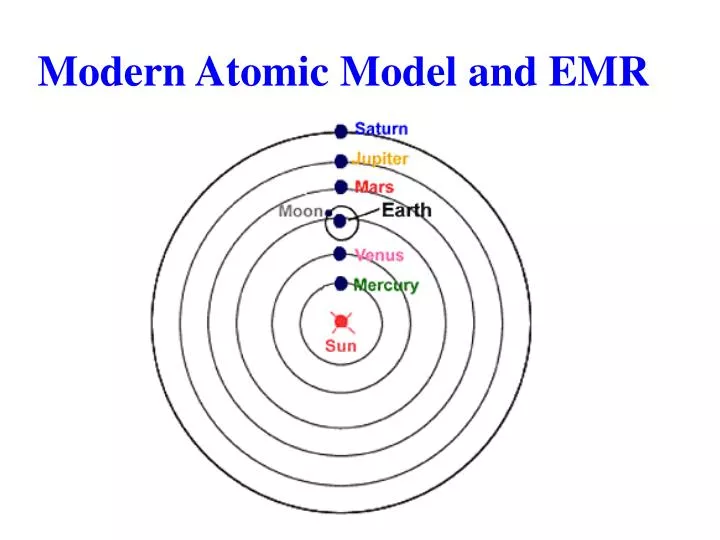
PPT Modern Atomic Model and EMR PowerPoint Presentation, free download ID4823594
To a certain extent modern atomic theory has bridged the gap between atomistic and holistic thought. Atomism - Modern Theory, Particles, Structure: With the development of a scientific atomic theory, the general philosophical problems gradually disappeared into the background. All attention is focused on the explanation of concrete phenomena.