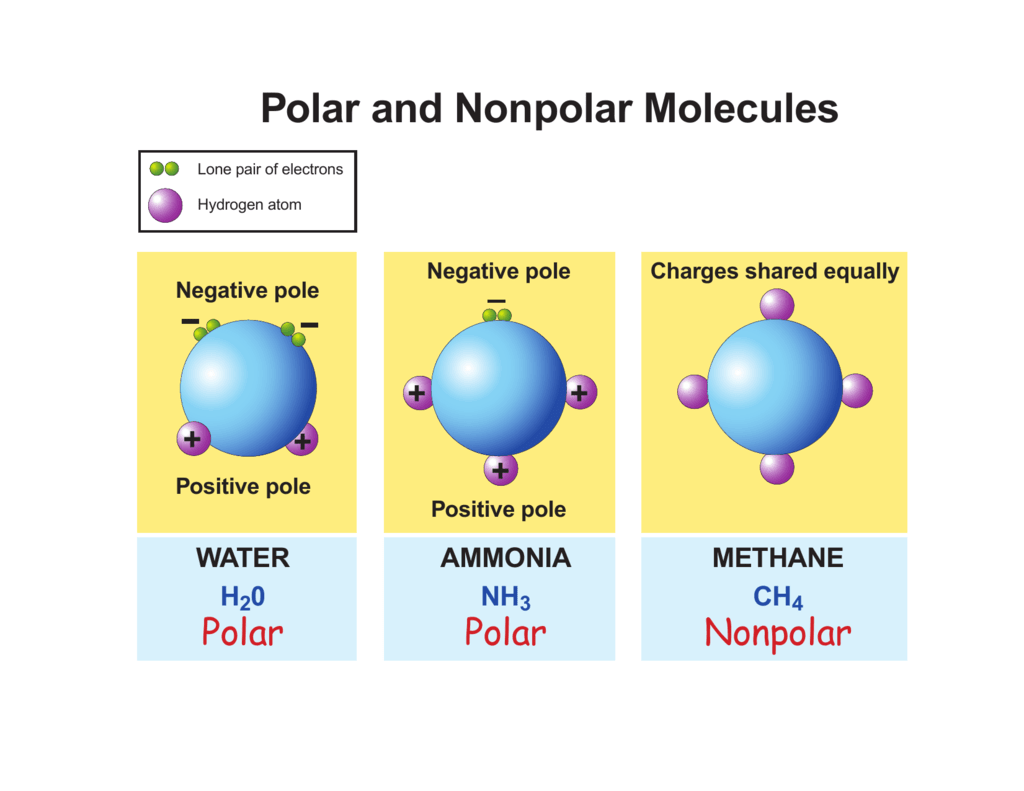
Polar and Nonpolar Molecules
When the difference is very small or zero, the bond is covalent and nonpolar. When it is large, the bond is polar covalent or ionic. The absolute values of the electronegativity differences between the atoms in the bonds H-H, H-Cl, and Na-Cl are 0 (nonpolar), 0.9 (polar covalent), and 2.1 (ionic), respectively.

Cl2 lewis structure, Molecular shape, Polar or NonPolar, Dot diagram
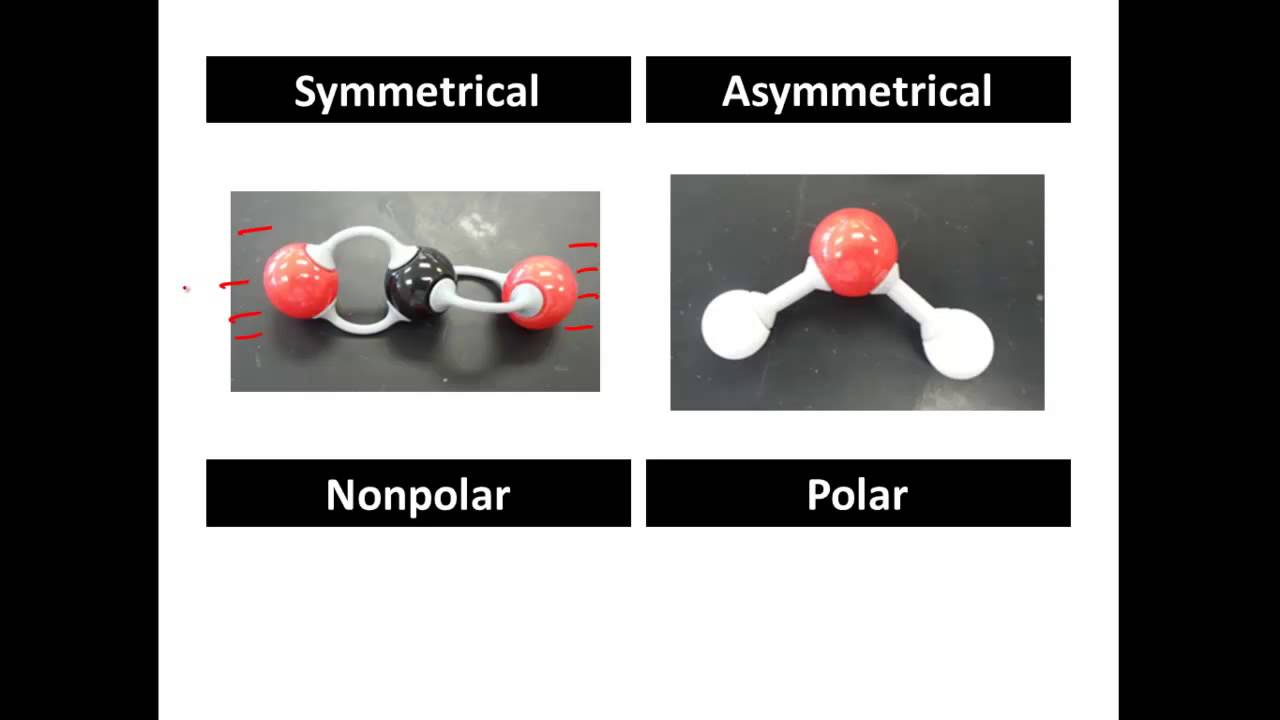
The molecule is symmetric. The two oxygen atoms pull on the electrons by exactly the same amount. Propane is nonpolar, because it is symmetric, with H atoms bonded to every side around the central atoms and no unshared pairs of electrons. Exercise 4.12. 1. Label each of the following as polar or nonpolar.

Best Explanation CH2Cl2 polar or nonpolar [N01] Science Education and Tutorials
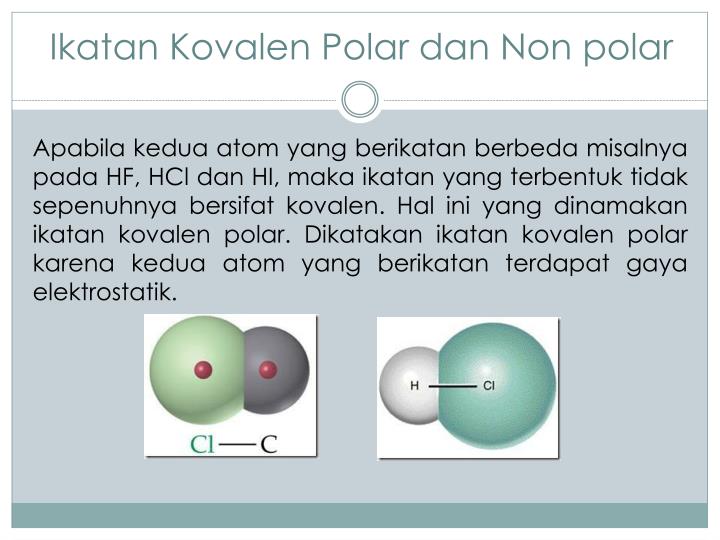
Depending on the difference in the electronegativity values, covalent bonds can be polar and nonpolar. If the difference in electronegativity is less than 0.5, the electrons are about equally shared between the two atoms, forming a nonpolar a covalent bond. If the difference in electronegativity is between 0.5 and 1.7, we have a polar covalent.

15 10 Contoh Senyawa Polar Dan Nonpolar
Figure 5.3.7: The molecular geometry of a molecule affects its polarity. In CO 2, the two polar bonds cancel each other out, and the result is a nonpolar molecule. Water is polar because its bent shape means that the two polar bonds do not cancel. Some other molecules are shown below (see figure below).

Elements Lewis Structure/ Sharing of Electrons Type of Bond (polar or nonpolar) Cl2 CHA
The covalent bond formed by the two atoms is said to be non-polar if the electronegativity of both atoms is equal. Note: It is also possible to have polar bonds within a non-polar molecule because the polarity of bonds gets canceled by each other due to symmetric geometrical shape. Few examples of nonpolar molecules are Hexane, CCl4, etc.
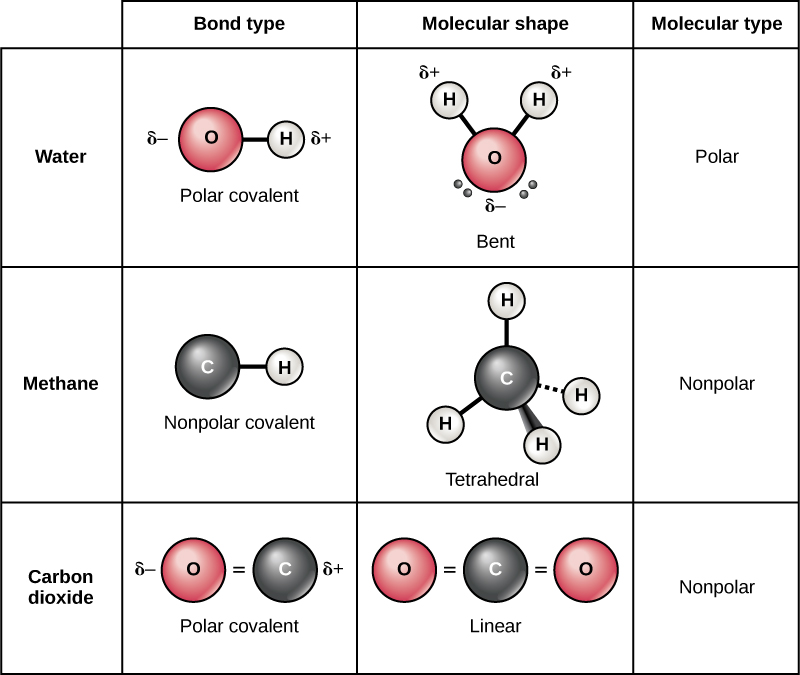
Difference Between Polar and Nonpolar Molecules Definition, Formation, Properties, Examples
Far West Laboratories, Inc. 6602 2nd Street. Riverbank, CA 95367. Phone: (209) 869-9260. Certificate No. Expiration Date. 1310 2/28/2022. *As of 1/1/2020, this list supersedes all previous lists for this certificate number. Customers: Please verify the current accreditation standing with the State.
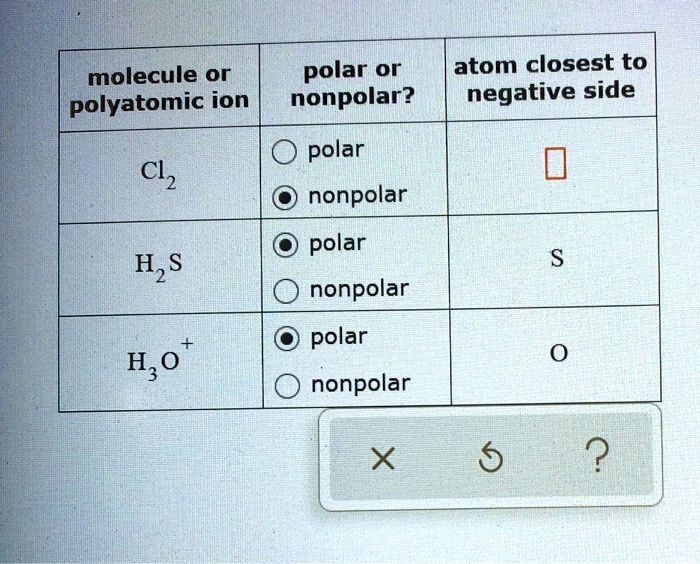
SOLVED molecule or polar or atom closest to polyatomic ion nonpolar? negative side polar Cl2
The whole community pigments and lipids have been examined during a 5-year period in two commercial solar salterns located in the United States and in Israel. There were significant differences in the complexity of the lipid and pigment patterns within the California saltern system, and these differ.

Polar Vs Nonpolar Ciencias exatas, Ciência química, Físicoquímica
5.10: Electronegativity and Bond Polarity is shared under a not declared license and was authored, remixed, and/or curated by LibreTexts. Covalent bonds can be nonpolar or polar, depending on the electronegativities of the atoms involved. Covalent bonds can be broken if energy is added to a molecule. The formation of covalent bonds is..

Is ClF Polar or Nonpolar (Chlorine Monoluoride) YouTube
View detailed information about property 4317 Polar Way Unit 24, Riverbank, CA 95367 including listing details, property photos, school and neighborhood data, and much more.
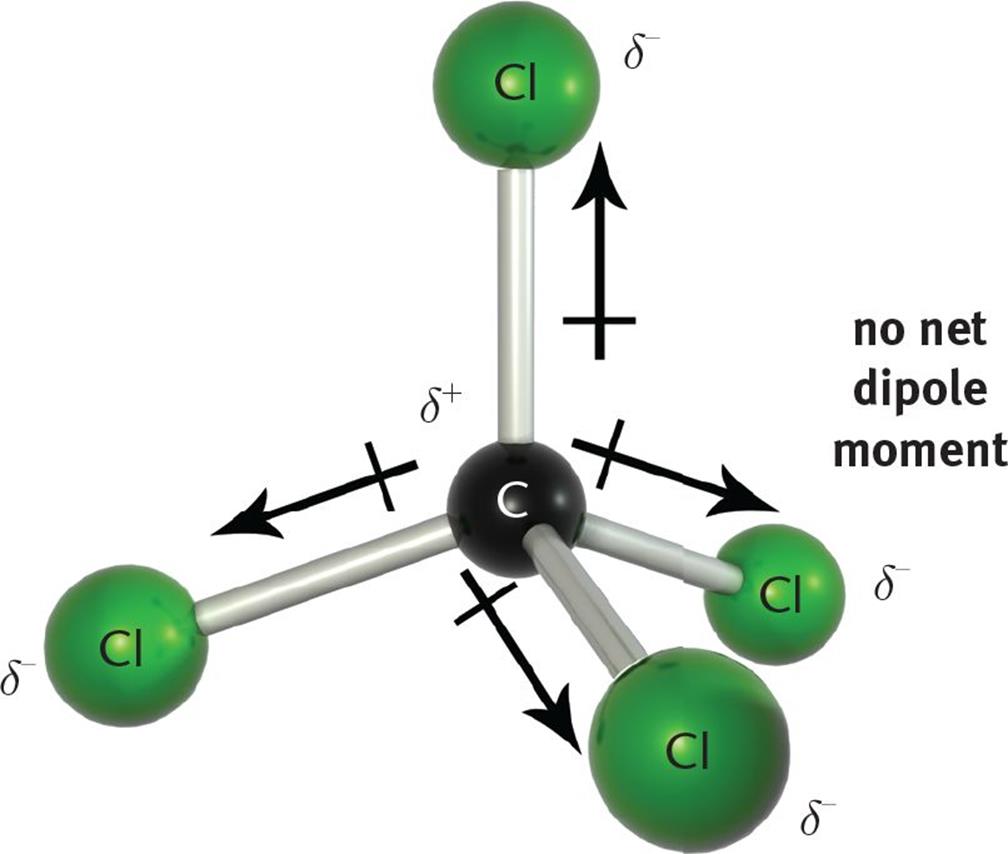
Simple Question Do all polar molecules have a dipole moment? r/chemhelp
Figure 6.2.1 (a) The distribution of electron density in the HCl molecule is uneven. The electron density is greater around the chlorine nucleus. The small, black dots in the center of the green spheres indicate the location of the hydrogen and chlorine nuclei in the molecule. (b) Symbols δ+ δ + and δ− δ − indicate the polarity of the H.

MakeTheBrainHappy Is Cl2 Polar or Nonpolar?
Hydrogen=2.2, carbon=2.5 and chlorine=3.1. So, electronegativity difference between C-H=0.3 and C-Cl=0.6. It proves that CH2Cl2 is polar but a moderate polar as the difference between their electronegativity is quite small. The total number of valence electrons in the CH2Cl2 molecule is 20. Carbon contains 4 valence electrons and hydrogen has 1.

🔴IKATAN KOVALEN POLAR & NON POLAR, 🔴SENYAWA KOVALEN POLAR DAN SENYAWA KOVALEN NONPOLAR YouTube
Learn to determine if CH2Cl2 (Dichloromethane) is polar or non-polar based on the Lewis Structure and the molecular geometry (shape).We start with the Lewis.
Polar and Nonpolar Molecules JourneyilSalinas
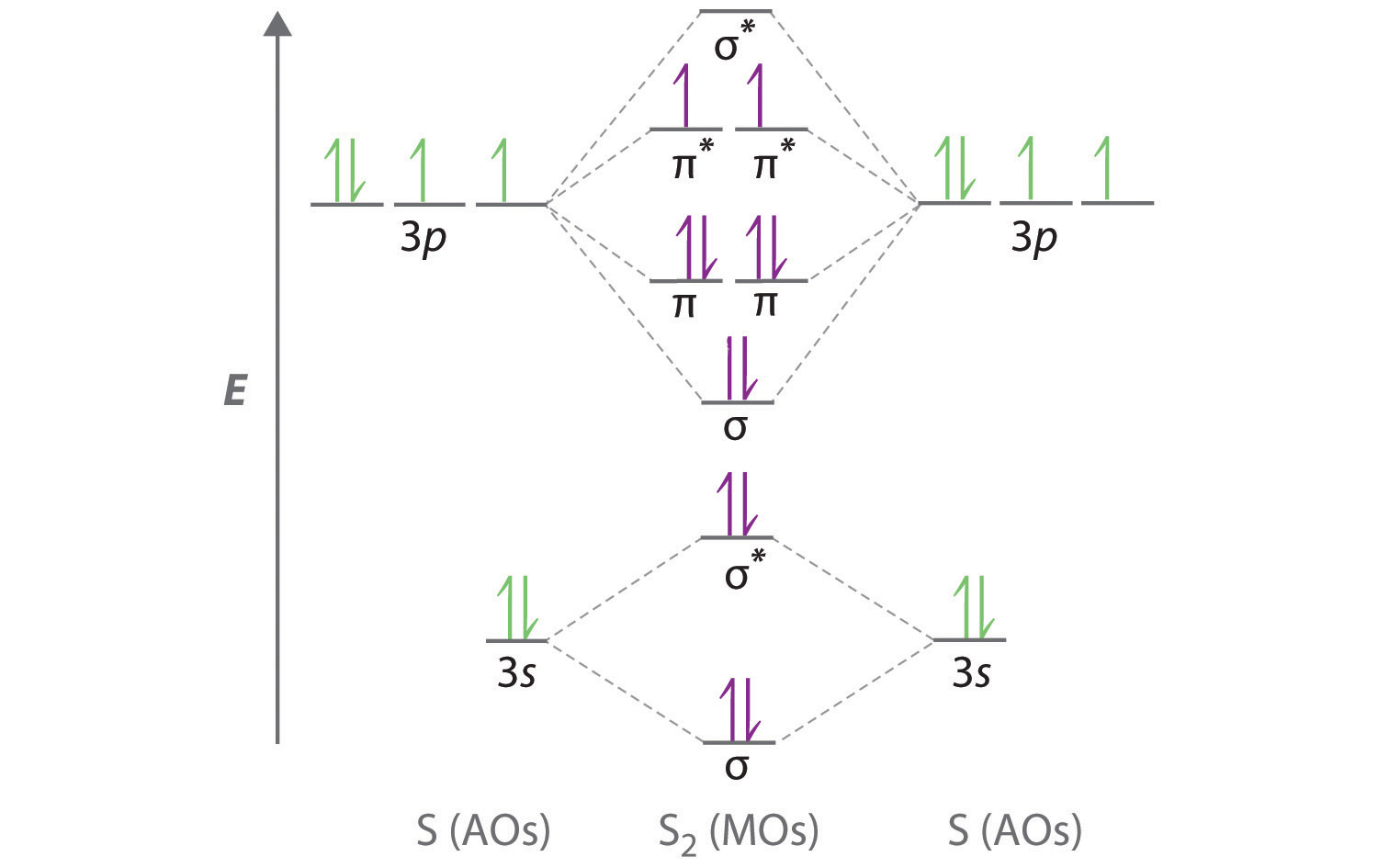
Cl2 (Chlorine) is nonpolar in nature because of its linear symmetrical shape and it consists of two chlorine atoms having equal electronegativity. As a result, both atoms have equal charge distribution on them, and the molecule results in zero dipole moment that makes the chlorine molecule nonpolar. Chlorine is a highly reactive element and.
Molecular Orbital Diagram For Cl2
The Cl-Cl bond is non-polar since the electronegativity difference is zero. The dipole moment of the bond comes out to be zero. In a diatomic molecule, a non-polar bond implies a non-polar molecule. Must Read: Is Cl2 Polar or Nonpolar. Is N2 Polar or Nonpolar . Preparation of Chlorine gas . Heating concentrated HCl with metal oxide
Ikatan Kovalen Polar Dan Nonpolar Beserta Contoh Ikatannya Rumus Kimia Sexiz Pix
The polarity of molecules is related to the polarity of bonds within the molecule, but just having polar bonds is not enough to create a polar molecule. Consider, for example, CCl 4 and CHCl 3. Carbon tetrachloride has 4 fairly polar bonds but they form a regular tetrahedron and the polarity of the individual bonds cancel each other out to.

Is Cl2 Polar or Nonpolar? Techiescientist
Let's dive into it! Cl2 is a NONPOLAR molecule because any two bonding atoms whose electronegativity difference value is less than 0.4 forms a nonpolar bond. Here in Cl2 molecule, both the atoms are Chlorine atoms. Because of this, the electronegativity difference of both the Chlorine atoms (Cl = 3.16) is 0 (i.e 3.16 - 3.16 = 0).