
PCl3 Molecular Geometry / Shape and Bond Angles YouTube
Key Points To Consider When Drawing The PCl3 Electron Dot Structure. A three-step approach for drawing the PCl3 Lewis structure can be used. The first step is to sketch the Lewis structure of the PCl3 molecule, to add valence electrons around the phosphorus atom; the second step is to add valence electrons to the three chlorine atoms, and the final step is to combine the step1 and step2 to get.

lewis structure and molecular geometry of PCl3? OpenStudy
The Lewis structure of PCl3 shows that phosphorus (P) is the central atom bonded to three chlorine (Cl) atoms. The central phosphorus atom has a lone pair of electrons and forms three single bonds with chlorine atoms. The Lewis structure helps in understanding the molecular geometry and chemical properties of PCl3.

Lewis Dot Diagram For Pcl3 General Wiring Diagram
Phosphorus trichloride is the precursor to organophosphorus compounds. It reacts with phenol to give triphenyl phosphite : 3 PhOH + PCl3 → P (OPh)3 + 3 HCl (Ph = C6H5) Alcohols such as ethanol react similarly in the presence of a base such as a tertiary amine: [9] PCl3 + 3 EtOH + 3 R3N → P (OEt)3 + 3 R3NH+Cl−.
35 Lewis Dot Diagram For Pcl3 Wiring Diagram List
Check me out: http://www.chemistnate.com

Structure Phosphorous Trichloride (PCL3), Grade Standard Technical
PCl3 Molecular Electron Geometry, Lewis Structure, Bond Angles and Hybridization. Phosphorus trichloride is made up of one Phosphorus atom and three Chlorine atoms, having a chemical formula of PCl3. It is a volatile liquid that reacts with water and releases HCl gas. It is a toxic compound but is used in several industries.

Lewis Dot Diagram For Pcl3 General Wiring Diagram
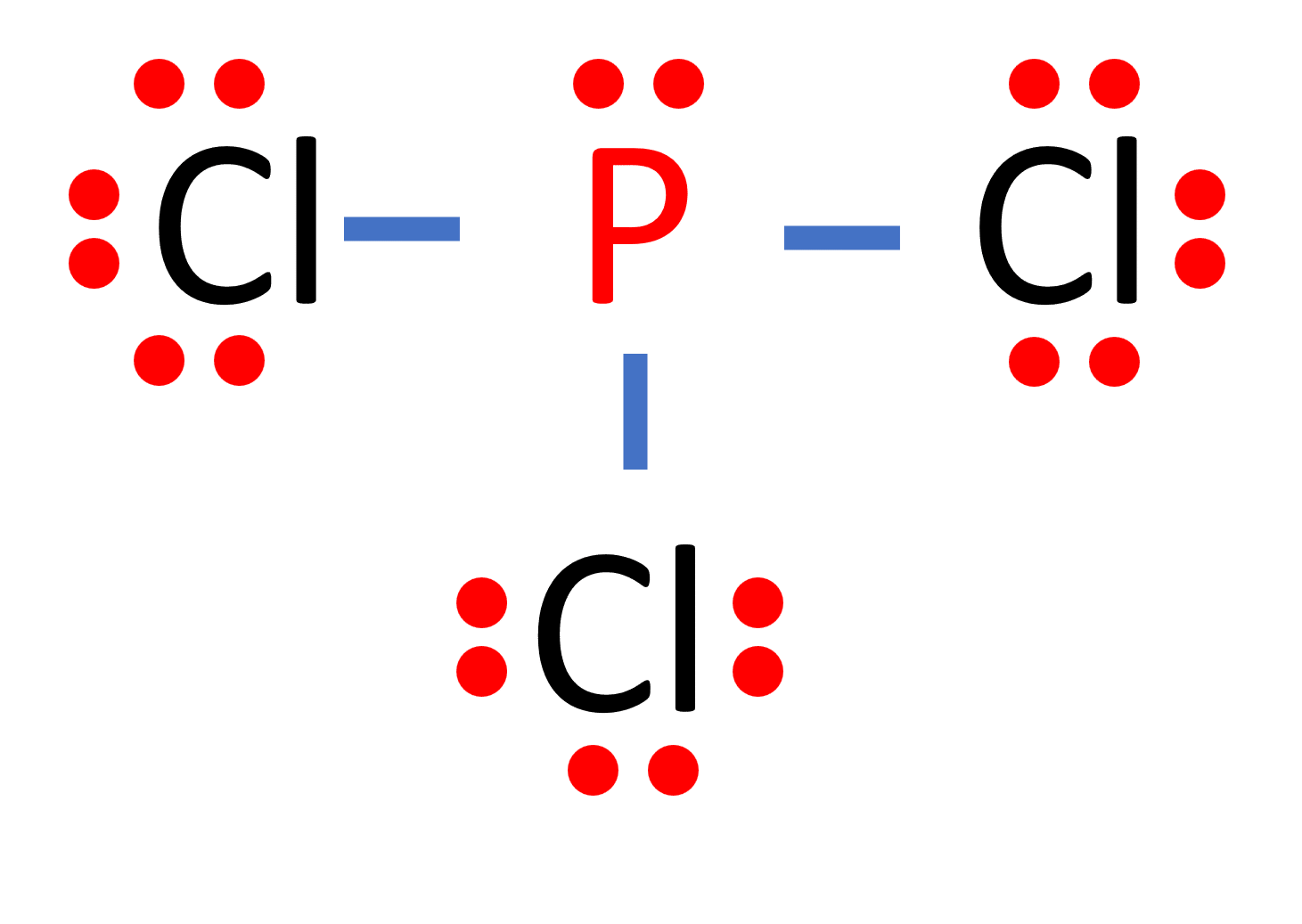
PCl 3 (Phosphorus Trichloride) Lewis Structure. Phosphorus trichloride (PCl 3) contains three chlorine atoms and one phosphorus atoms. In PCl 3 lewis structure, each chlorine atom is joint with center phosphorus atom through a single bond. Also, there is a lone pair on phosphorus atom. In this tutorial, we will learn how to draw the lewis structure of PCl 3 with all theories.
PCL3 Molecular Electron Geometry, Lewis Structure, Bond Angles and
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".
PCl3 Lewis Structure in Four Simple Steps What's Insight
Step #1: Calculate the total number of valence electrons. Here, the given molecule is PCl3 (phosphorus trichloride). In order to draw the lewis structure of PCl3, first of all you have to find the total number of valence electrons present in the PCl3 molecule. (Valence electrons are the number of electrons present in the outermost shell of an.

Media Portfolio
A step-by-step explanation of how to draw the PCl3 Lewis Dot Structure (Phosphorus Trichloride).For the PCl3 structure use the periodic table to find the tot.
New Vsepr Pcl3 Molecular Geometry PNG GM
PCl 3 Lewis structure. PCl 3 (phosphorus trichloride) has one phosphorus atom and three chlorine atoms. In the PCl 3 Lewis structure, there are three single bonds around the phosphorus atom, with three chlorine atoms attached to it. Each chlorine atom has three lone pairs, and the phosphorus atom has one lone pair.

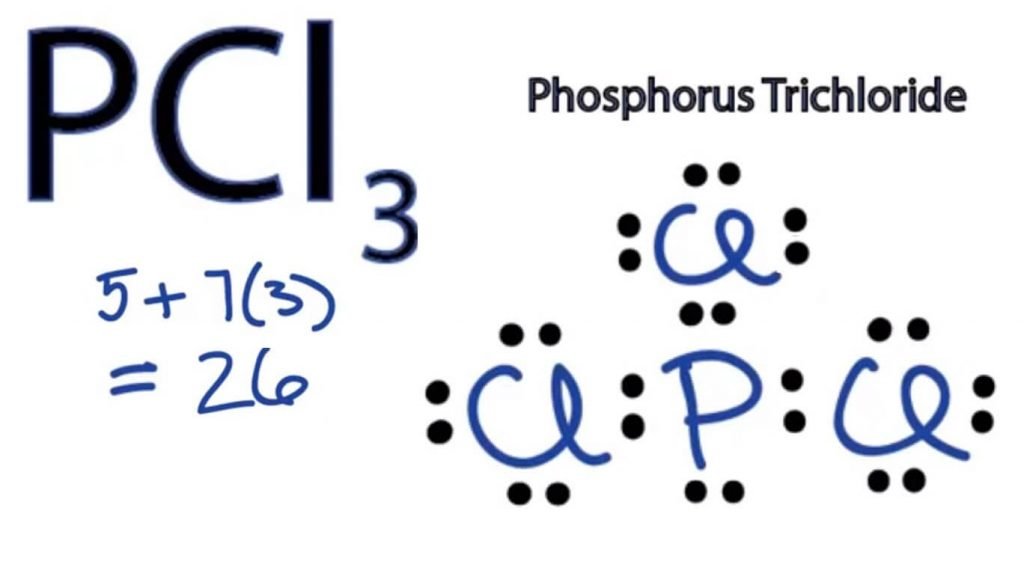
So far, we’ve used 26 of the PCl3 Lewis structure’s total 26 outermost
A quick explanation of the molecular geometry of PCl3 including a description of the PCl3 bond angles.Looking at the PCl3 Lewis structure we can see that the.

PCl3 Lewis StructureLewis Structure of PCl3 (Phosphorus Trichloride
3.1: Lewis Structures. Chemical bond refers to the forces holding atoms together to form molecules and solids. This force is of an electric nature, and the attraction between electrons of one atom to the nucleus of another atom contributes to what is known as chemical bonds.

What Is Pcl3 Lewis Structure?
Steps of drawing PCl3 lewis structure Step 1: Find the total valence electrons in PCl3 molecule. In order to find the total valence electrons in PCl3 molecule, first of all you should know the valence electrons present in phosphorus atom as well as chlorine atom. (Valence electrons are the electrons that are present in the outermost orbit of any atom.).
Lewis Dot Diagram For Pcl3 General Wiring Diagram
Phosphorus pentachloride is the chemical compound with the formula PCl 5. It is one of the most important phosphorus chlorides/oxychlorides, others being PCl 3 and POCl 3. PCl 5 finds use as a chlorinating reagent. It is a colourless, water-sensitive solid, although commercial samples can be yellowish and contaminated with hydrogen chloride .
[Solved] what is the lewis structure, electron geometry, molecular
PCl3 Lewis Structure, Hybridization, Molecular Geometry, and MO Diagram. Phosphorus trichloride with a chemical formula PCl3 is a yellow fuming liquid. This liquid can be colorless as well. PCl3 is a toxic liquid with an unpleasant smell. The molar mass of this compound is 137.33 g/mol.

How to draw PCl3 Lewis Structure? Science Education and Tutorials
I quickly take you through how to draw the Lewis Structure of PCl3, phosphorous trichloride. I also go over hybridization, shape and bond angle.