
A general schematic of an accelerator mass spectrometer. Ions from the... Download Scientific
Accelerator mass spectrometry (AMS) is an analytical method used to detect the amount of radioactive carbon in a biological sample. It is an extremely sensitive methodology that can be used in early clinical research when conventional radiometric detection methods such as liquid scintillation counting are not possible.
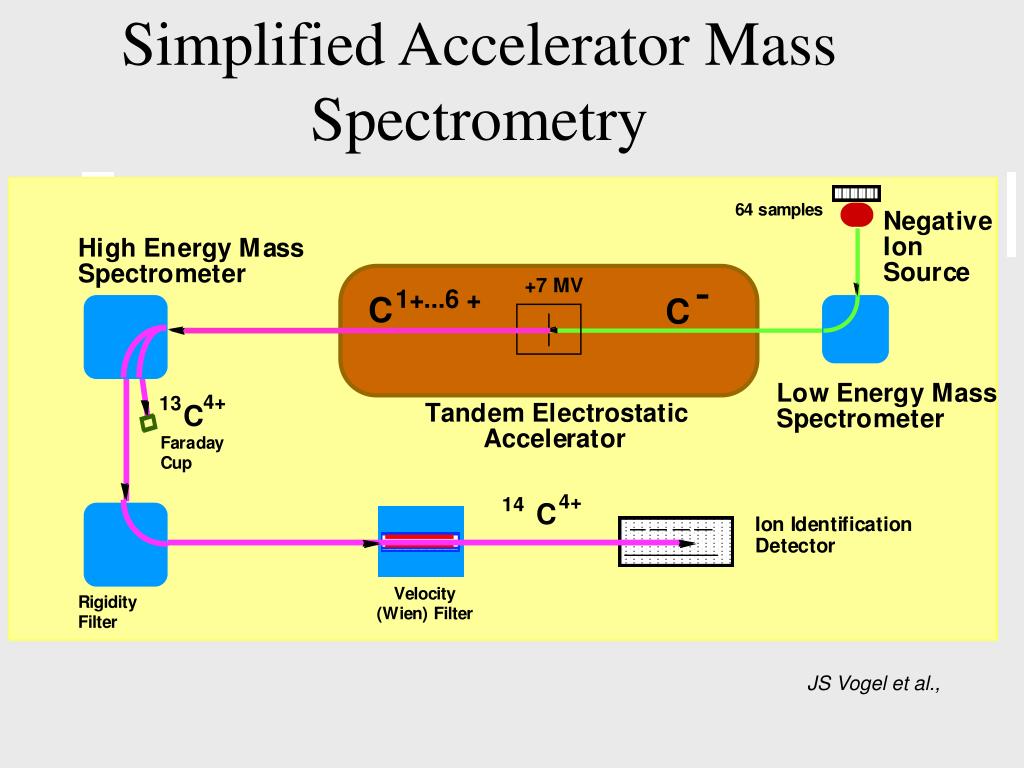
PPT Lecture 5 Radiocarbon PowerPoint Presentation, free download ID4317104
Stage 2: Acceleration: The ions are accelerated so that they all have the same kinetic energy and directed into a mass analyzer. Stage 3: Separation according to the mass-to charge-ratio (m/ze) of the ions: The ions are sorted according to their (m/ze). Stage 4: Detection: The beam of ions passing through the mass analyzer is detected as a current.
Determining radioactive waste with accelerator mass spectrometry GRS gGmbH
Accelerator Mass Spectrometry. AMS is an analytical technique that can quantify long-lived isotopes with attomole (amol) (10 − 18) sensitivity in isotope-labeled drugs and toxicants. AMS counts atoms of a rare isotope of interest and reports the ratio of the counted isotope to that of the total number of atoms of the element.
Lancaster Accelerator Mass Spectrometer (LAMSUK) National Nuclear User Facility
An accelerator mass spectrometer measures the amounts of different isotopes within a sample. For carbon dating, the process starts in an ionizing chamber, where sample carbon atoms are given a negative charge and initially accelerated to an injection magnet where the particle beam is split by mass and sequentially injected into the accelerator.

Accelerator Mass Spectrometry AMS Analysis Measurlabs
PDF Tools Share Abstract In this overview the technique of accelerator mass spectrometry (AMS) and its use are described. AMS is a highly sensitive method of counting atoms. It is used to detect very low concentrations of natural isotopic abundances (typically in the range between 10 −12 and 10 −16) of both radionuclides and stable nuclides.

Accelerator Mass Spectrometer Stock Image C029/0701 Science Photo Library
Accelerator mass spectrometry ( AMS) is a form of mass spectrometry that accelerates ions to extraordinarily high kinetic energies before mass analysis.
A typical accelerator mass spe [IMAGE] EurekAlert! Science News Releases
Introduction. Accelerator mass spectrometry (AMS) was developed for analyzing 14 C in environmental and archeological specimens in the 1970s (although it was first demonstrated in the 1930s). It is now principally used to measure only a handful of isotopes, although it is feasible to apply it to many additional analytes.

How the accelerator mass spectrometer works The Channel
Accelerator mass spectrometry ( AMS) is a powerful method for the measurement of very low abundance nuclei even in a background of much stronger isobars. The technique at ATLAS is applicable to many rare nuclei (including very low abundance nuclei 10 -9 to 10 -16) for many scientific goals, but one of the most important areas of research at.

André E. Lalonde Accelerator Mass Spectrometry (AMS) Laboratory
There are two accelerator systems commonly used for radiocarbon dating through accelerator mass spectrometry. One is the cyclotron, and the other is a tandem electrostatic accelerator. [VIDEO] AMS vs Radiometric Techniques AMS Analysis via Tandem Accelerator
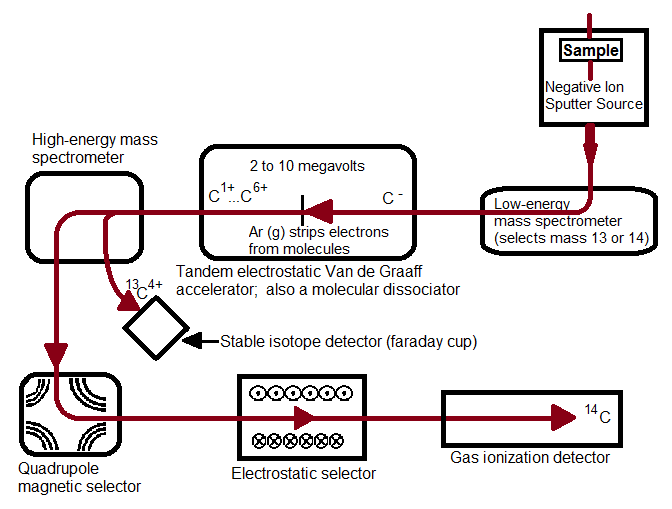
Accelerator Mass Spectroscopy Chemistry LibreTexts
Accelerator Mass Spectrometry (AMS) adds the techniques of higher energy charged particle acceleration to the basic principles of Isotope Ratio Mass Spectrometry (IRMS) to provide extremely low detection capability (below 1 femtogram) of rare isotopes in samples of natural materials as small as 1 mg. Depending on the element selected and the con.

How radiocarbon dating is done? Digitash
Accelerator mass spectrometry (AMS) is a mass-spectrometric method using entire accelerator systems to measure ultralow traces of long-lived radioisotopes. AMS spectrometers produce an ion beam from a sample of interest and separate ions according to their magnetic, electric, and atomic characteristics.

A schematic of the 200 keV 'MICADAS' accelerator mass spectrometry... Download Scientific Diagram
Accelerator Mass Spectroscopy. AMS requires a particle accelerator, originally used in nuclear physics research, which limits its widespread use due to high costs and technical complexity. Fortunately, UC Davis researchers have access to the Lawrence Livermore National Laboratory Center for Accelerator Mass Spectrometry (CAMS LLNL), one of over.

Accelerator Mass Spectrometry Radiocarbon Dating Telegraph
Accelerator mass spectrometry (AMS) is sometimes called 'the art of counting atoms one by one'. In addition to counting individual atoms, AMS is also capable to determine both mass number (A) and atomic number (Z).

Accelerator mass spectrometry research Stock Image C009/8195 Science Photo Library
One of the applications of nuclear physics techniques that is listed in Table 1, and which has been of great benefit to other fields of scientific endeavor, is accelerator mass spectrometry (AMS). The capability of AMS in extremely sensitive radioisotope measurements has been extensively demonstrated over the past 30 years.

The section of the accelerator mass spectrometer (AMS Stock Photo, Royalty Free
1. Introduction. Accelerator mass spectrometry (AMS) is arguably the most sensitive method to measure long-lived radionuclides with isotopic abundances as low as 10 −12 to 10 −16.It should be noted at the onset that the term 'long-lived' has a different meaning in geochronology [1], where long-lived radionuclides are the ones which survive the age of the solar system (e.g. 232 Th, 238.

Accelerator mass spectrometry hires stock photography and images Alamy
Accelerator mass spectrometry (AMS) is a method of analysis incorporating particle accelerator technology into a mass spectrometer. The field was developed in 1977 (Bennett et al., 1977; Muller, 1977; Nelson et al., 1977) as an analytical tool first for the measurement of radiocarbon ( 14 C) and it was quickly extended to other radionuclides.