Risk Management SOP
This SOP defines the approach to Quality Risk Management (QRM) of a GMP site and gives practical examples for tools which may be used to facilitate the process and to aid personnel performing the assessment. 2.0 Scope Applicable to any process at a GMP site which requires a Risk Management approach.

FREE 61+ SOP Templates in PDF MS Word
• ensure that a quality risk management process is defined, deployed, and reviewed and that adequate resources are available. B. Initiating a Quality Risk Management Process (4.2) Quality risk management should include systematic processes designed to coordinate, facilitate and improve science-based decision making with respect to risk.

Quality risk management sop
This Master Plan defines how our Quality Risk Management (QRM) program will be conducted through integration of knowledge gained from formal risk assessments, operational alerts, change controls and inspections as required by ICH Q9 Quality Risk Management .

SOP for Risk Management according to EN ISO 14971
Quality risk management is a systematic process for the assessment, control, communication, and review of risks to the quality of the drug (medicinal) product across the product lifecycle. A model.
SOP for Quality Risk Management (Guideline ICH Q9) Pharma Beginners
Aug 1st 2023 SOP Templates: Guide to Safe Operating Procedures Template A Safe Operating Procedure (SOP) serves as detailed instructions outlining how to perform a task or activity safely. SOPs are useful for any routine, repeated process and are crucial for maintaining workplace health and safety.

Quality risk management sop
Clinical Risk Management SOP. 2. Clinical risk management plan. 3. Vendor oversight SOP. 4. Vendor oversight plan. 5. Clinical risk log. Clinical Risk Management SOP. This SOP template provides a framework for risk identification, classification, monitoring, and mitigation throughout the life cycle of a clinical trial as well as evaluation of.

QUALITY RISK MANAGEMENT SOP Template PH37 GMP, QSR & ISO Comp
Pharmaceutical Standard Operating Procedure Template- Describes the company's process to be used in conducting a risk assessment. This document provides principles and examples of tools for quality risk management that can be applied to different aspects of pharmaceuticla quality. These aspects include developemnt, manufacturing, distribution.
SOP for Quality Risk Management (Guideline ICH Q9) Pharma Beginners (2022)
This file is a template for a Master Plan that can be used by companies to define how their Quality Risk Management (QRM) program will be conducted through integration of knowledge gained from formal risk assessments, operational alerts, change controls and inspections as required by ICH Q9 Quality Risk Management.

quality risk management sop.docx Scatter Plot Production And Manufacturing
Keywords: Risk assessment, quality risk management, risk, harm, hazard, failure mode and effects analysis (FMEA) Current version - effective from 26/07/2023 International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) guideline Q9 (R1) on quality risk management - Step 5 - Revision 1
Quality Risk Management Sop Template Ph37 Gmp Qsr Iso Comp
SOP for Quality Risk Management 1.0 PURPOSE: This Standard Operating Procedure (SOP) establishes uniform requirements for quality risk management (QRM) utilizing a risk-based systems approach for implementation into a quality system. The Quality Risk Management process shall be based on scientific methodologies and practical decisions.

SOP for Quality Risk Management _ Pharmaceutical Guidelines Risk Management Risk Assessment
QUALITY RISK MANAGEMENT Q9(R1) INTERNATIONAL COUNCIL FOR HARMONISATION OF TECHNICAL REQUIREMENTS FOR PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED GUIDELINE QUALITY RISK MANAGEMENT Q9(R1) Draft version Endorsed on 18 November 2021 Currently under public consultation

SOP for Quality Risk Management Pharmaceutical Guidelines
The guidance replaces the draft guidance "Q9 (R1) Quality Risk Management" issued on June 15, 2022. The purpose of this guidance is to offer a systematic approach to quality risk management.
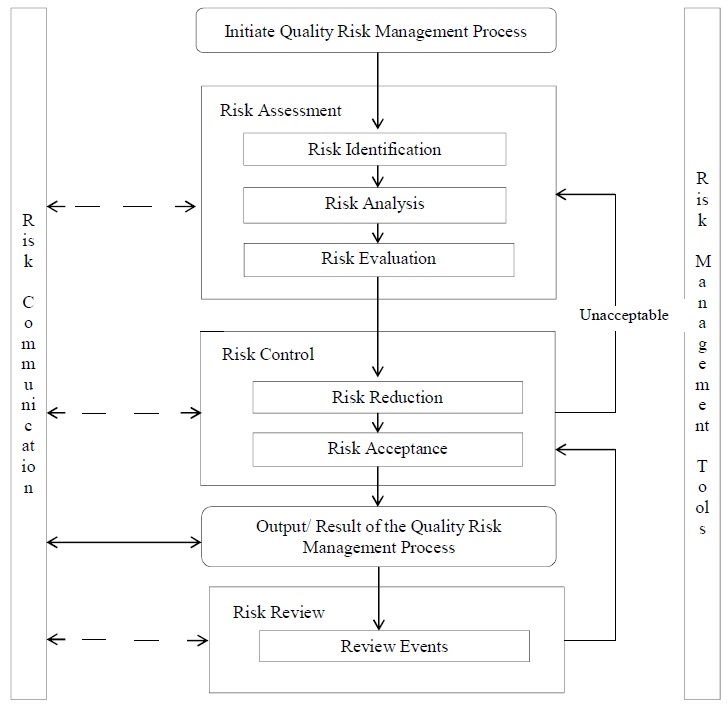
SOP for Quality Risk Management (Guideline ICH Q9) Pharma Beginners (2022)
Follow these steps to effectively utilize the QMS SOP Template in ClickUp: 1. Customize the template. Start by customizing the QMS SOP Template to fit your organization's specific needs. Review the existing content and make necessary adjustments to align with your company's quality standards and processes.
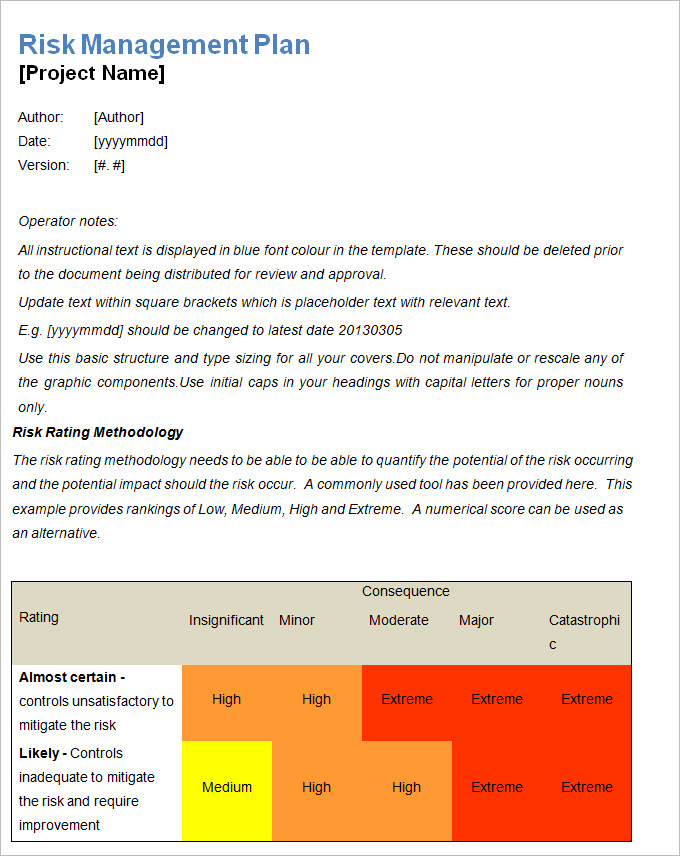
Risk Management Plan Templates 16+ Free Word, Excel & PDF Formats, Samples, Examples, Designs
Quality Manager, Risk Manager, Process Owners, Risk Owners, experts relevant to risk management. Actions to Address Risks and Opportunities Quality system procedure: Fills the Major gap in ISO 9001:2008 QSM documentation when transitioning to the new version of ISO 9001:2015.

Sop For Management Of Risk & Opportunity Risk Management Policy
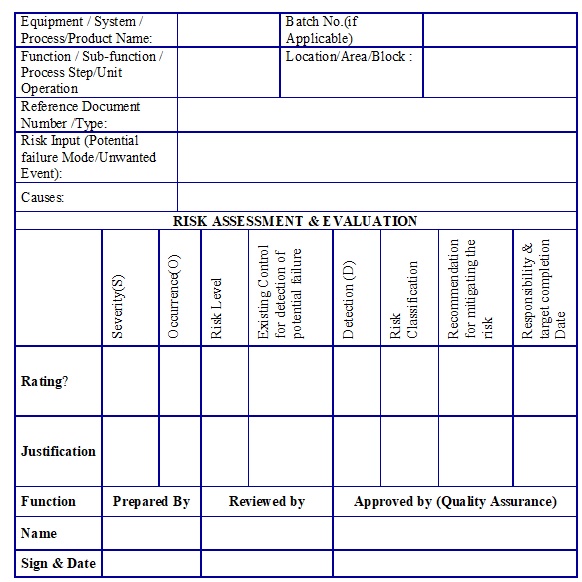
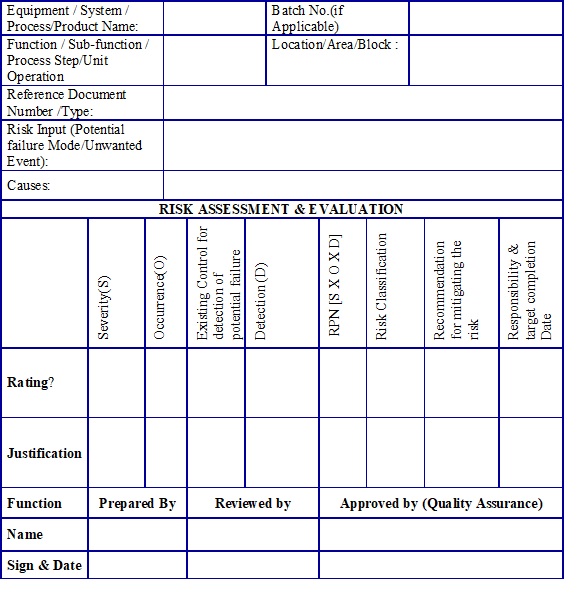
the level of effort, formality and documentation of the Quality Risk Management process should be commensurate with the level of risk. Risk Assessment: The Risk Assessment consists in the identification of hazards, analysis and evaluation of risks associated with exposure to those hazards Risk assessment defines with three fundamental questions.
Quality Risk Management Sop Template Ph37 Gmp Qsr Iso Comp
SOP on Quality Risk Management By Pharma pathway - March 21, 2017 0 24049 1.0 Objective To describe the procedure for management of risks, arising from different operations, activities and discrepancies. 2.0 Scope