
Quality System DocumentationPresentationEZE
A quality management system (QMS) is defined as a formalized system that documents processes, procedures, and responsibilities for achieving quality policies and objectives. A QMS helps coordinate and direct an organization's activities to meet customer and regulatory requirements and improve its effectiveness and efficiency on a continuous.
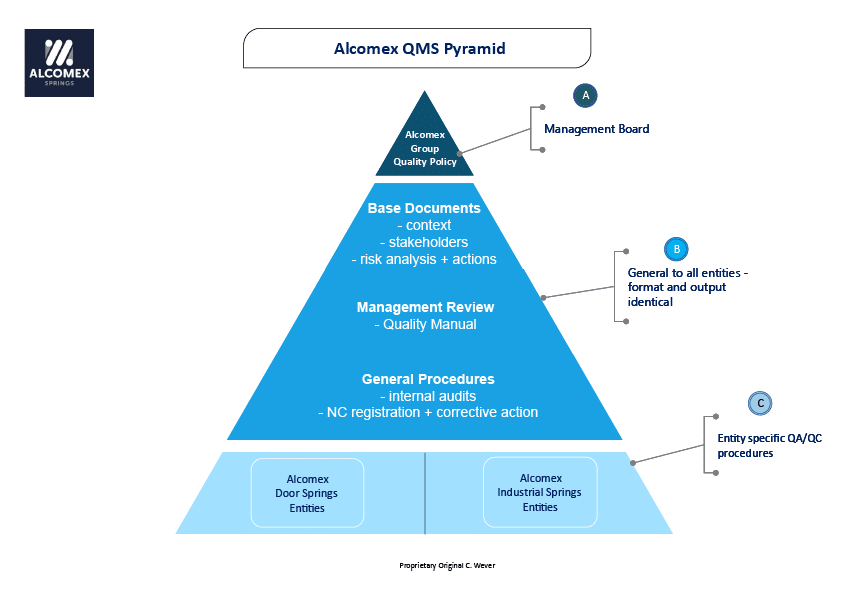
The Quality Management System pyramid news
Clause 7.5.1 General explains that the quality management system documentation shall include: documented information required by this International standard; documented information determined by the organization as being necessary for the effectiveness of the quality management system
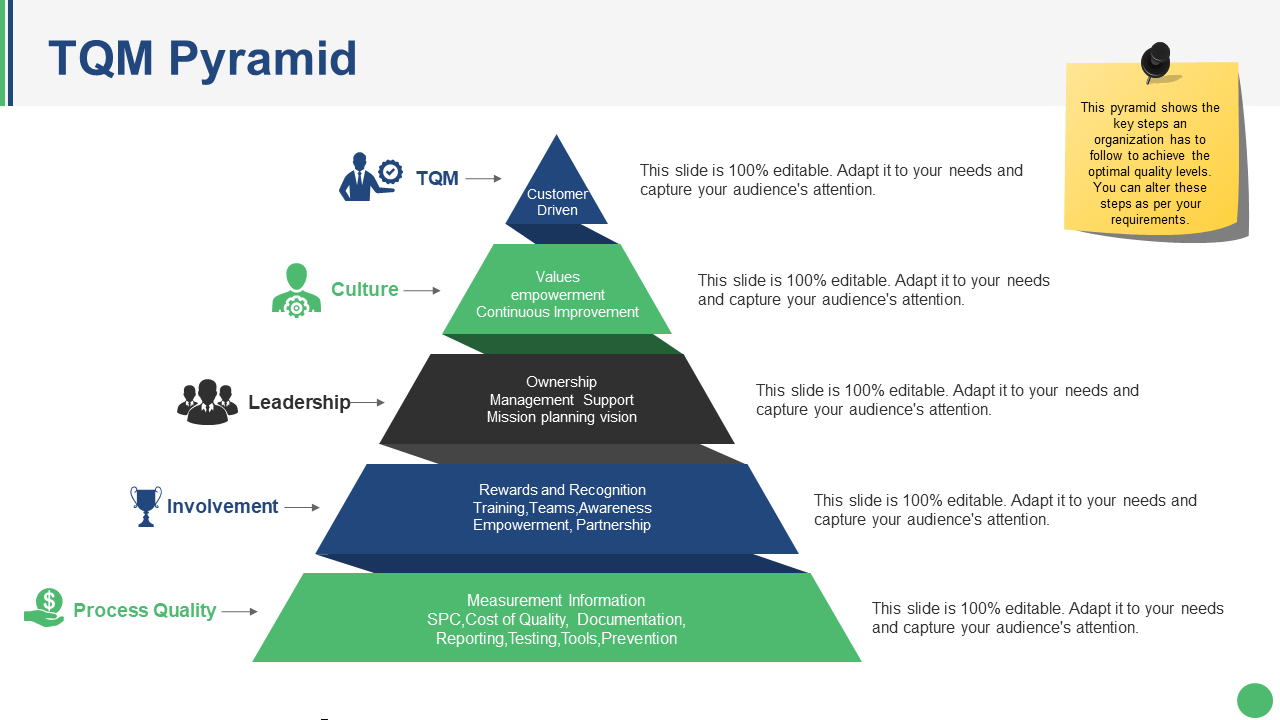
21 High Quality Templates For Your Quality Management
Previous Article. A Quality Management System (QMS) is a formalized system documenting processes, procedures, and responsibilities for ensuring consistent delivery of high-quality products and services to the consumer. A QMS provides a framework for employees to plan their work, monitor progress, identify problems, and take corrective action.
The Importance of Document Control a Complete Overview Nimonik Inc.
Abstract. Three quality systems that can be used in blood establishments are briefly explained. The Pyramid model is described as a tool to manage the quality systems. Finally, some experiences in.

GMP Document Management Documentation Hierarchy API FIRST
The quality manual is the document that identifies and describes the QMS. The adoption of a QMS should be a strategic decision of an organization. The design and implementation of an organization's QMS (documentation) is influenced by 1. its organizational environment, changes in that environment, and the risks associated with that environment,
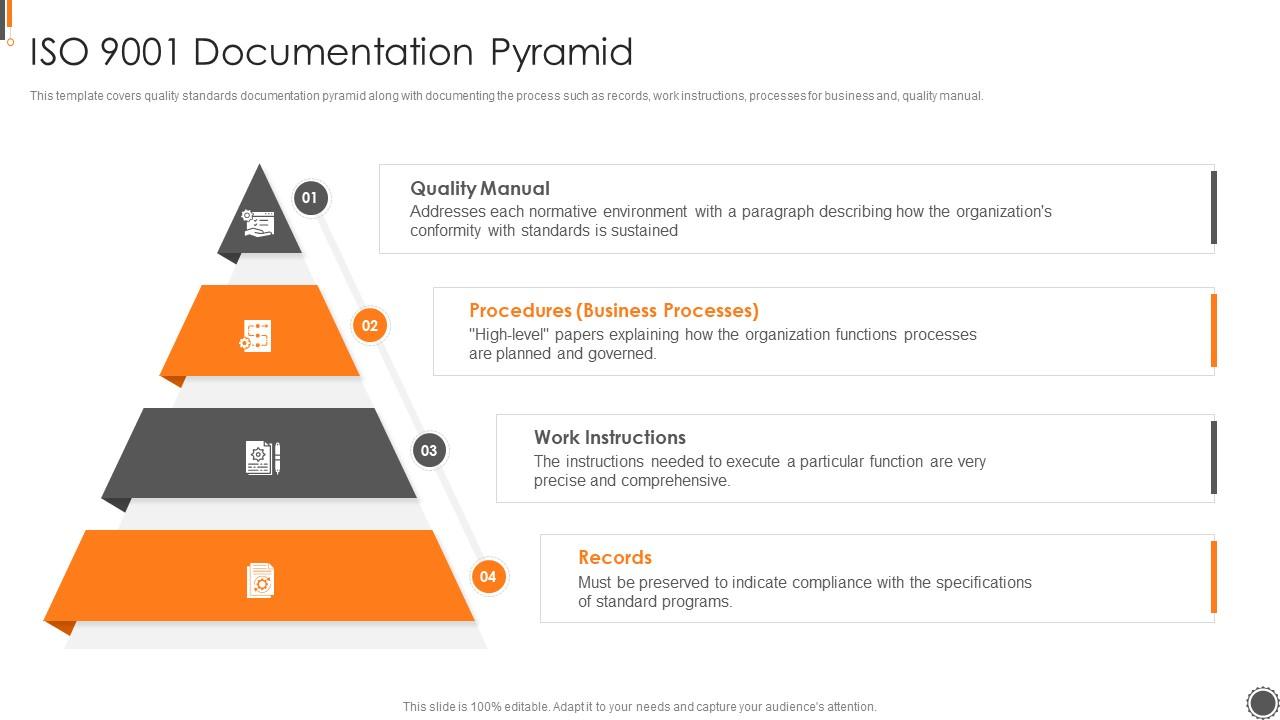
ISO 9001 Documentation Pyramid ISO 9001 Certification Process Ppt Sample Presentation Graphics
Article Full-text available Dec 2010 Milan Eri Vladimir Nedic Miladin Z Stefanovic Marko Djuki One of the basis of a series of standards JUS ISO 9000 is quality system documentation. An.
ISO 9001 QMS documentation How to structure it
Here's how to structure your IAF 16949 Quality Management System documentation: 1) Quality manual. The quality manual needs to be tailored to your company - its structure and content should be based on your company size, operational complexity, and employee competence. Smaller companies will be able to fit their whole QMS into one manual.

PPT Quality Management System and the Business Plan PowerPoint Presentation ID5706863
Pyramid of the quality system documentatio n This is achieved by the following measures and activities: Manage documents on the basis of written procedures Ensuring that the valid document.
How to Implement a QMS and Achieve ISO 9001 Certification
The solution is Lean. © 2020 Lean ISO Management Systems (Click on image to enlarge.) Businesses typically document their management system structure as a pyramid. Most of the manuals I have seen contained a section called "Documentation Structure."
Quality Management System Pyramid
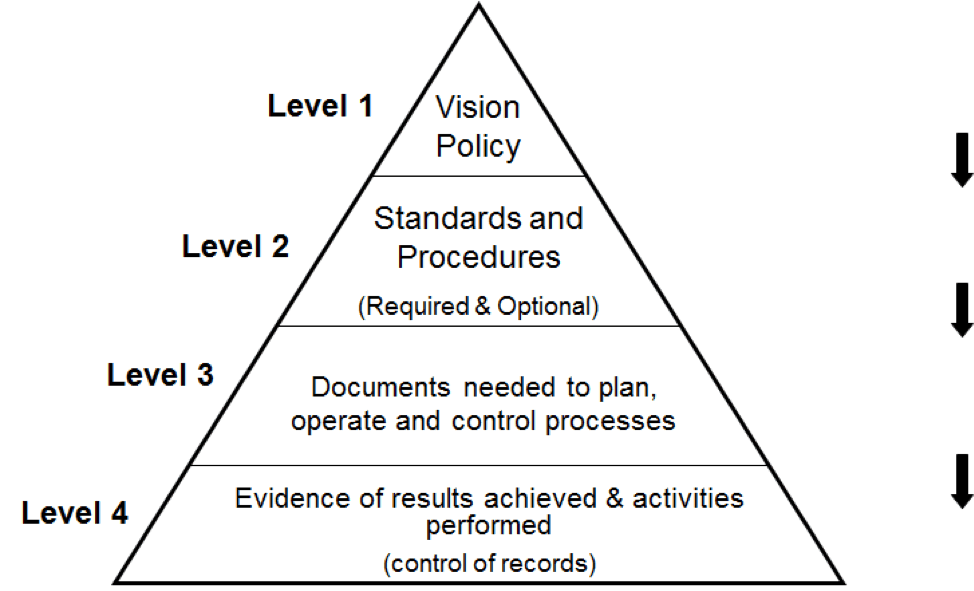
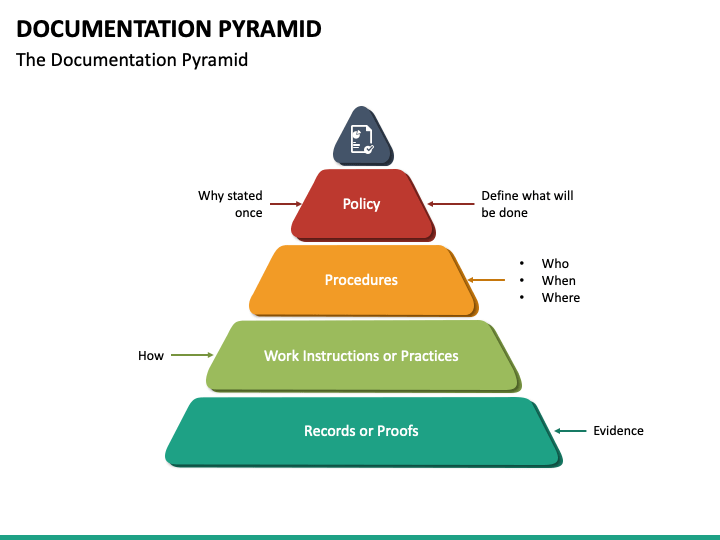
The QMS documentation hierarchy is presented in the diagram below: ISO 9001 requires different types of documents however, they do not need to be written as separate documents. The standard is flexible, so that the organization can decide on the size of the documents and the level of details documented.
Priority Pyramid For Total Quality Management Presentation Graphics Presentation PowerPoint
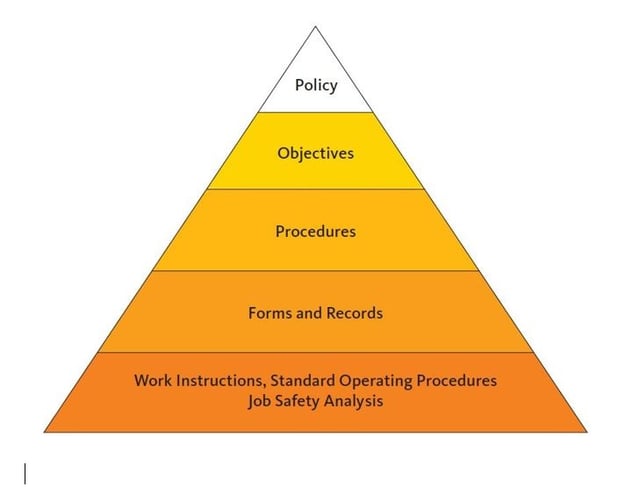
08 Oct 2021. One of the critical questions you may ask yourself or the expert creating your quality management system is about the various types of documentation involved in the whole system. There are few main document types within a standard QMS: Policy, Process, Procedure, and Work Instruction (sometimes called SOP - Standard Operation.

Quality Management System Documentation Quality Gurus
implement, and maintain a quality management system and continually improve its effectiveness in accordance with the requirements of this International Standard" Clause 4.2.1 General explains that the quality management system documentation shall include: a) documented statements of a quality policy and quality objectives; b) a quality manual
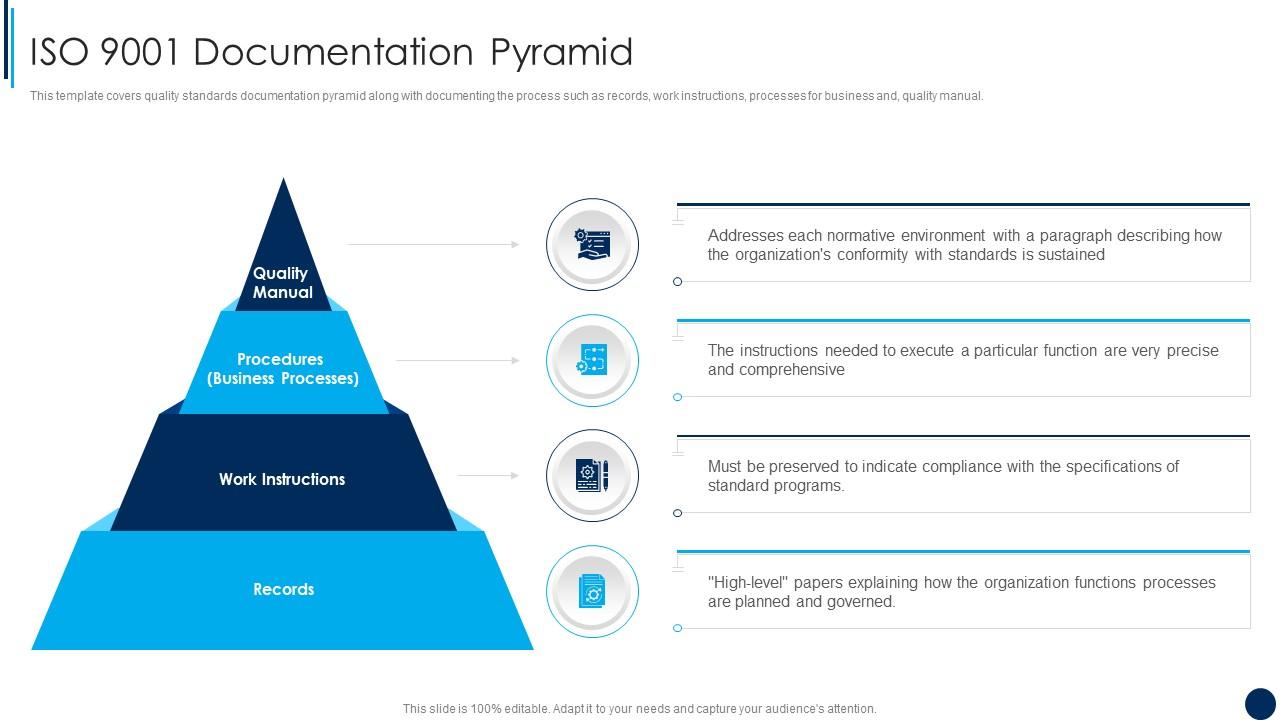
ISO 9001 Documentation Pyramid ISO 9001 Quality Management Ppt Demonstration Presentation
The Pyramid model is described as a tool to manage the quality systems. Finally, some experiences in other countries are given to prove the validity of the system. Keywords: Pyramid model, quality system, quality assurance, Good Manufacturing Practice (GMP), ISO 9001, GMP for blood banks, quality policy and strategy Go to: Introduction
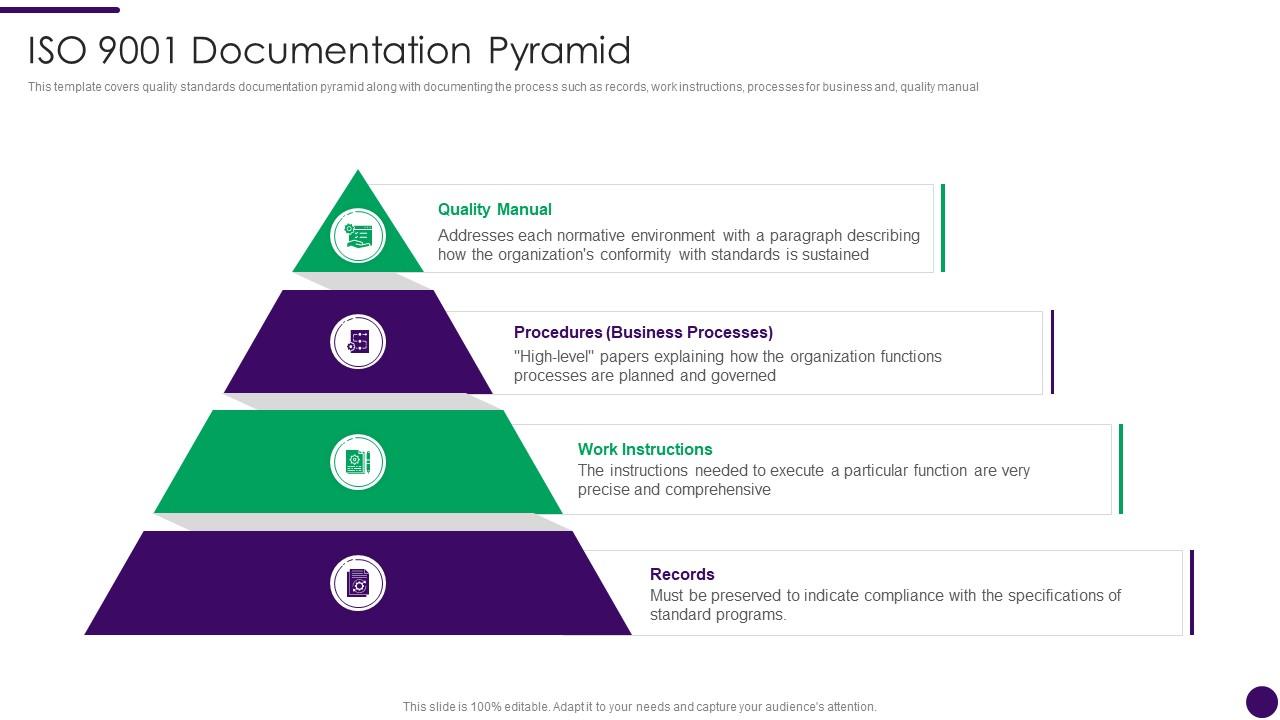
ISO 9001 Documentation Pyramid How To Achieve ISO 9001 Certification Ppt Ideas Presentation
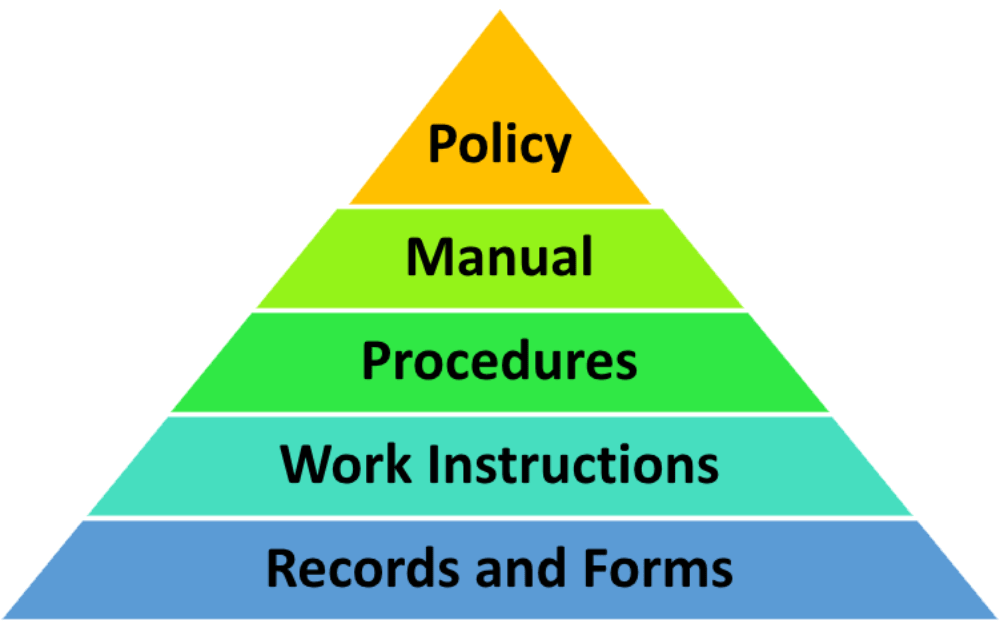
Quality System Documentation is a pyramid in shape. The Quality Manual, the top level document, links the quality system procedures, work instructions and forms via applicable document references. Be aware of when a company is audited, the auditors will use your Quality Manual as the audit document. The drawing shown below provides the general.

Quality Priority Pyramid Brewers Association
A Quality Manual Documented Procedures Work Instructions With ISO 9001:2015, it is now left up to the organization to determine which documents it believes it needs (considering factors such as "interested parties," "organizational knowledge," etc.).
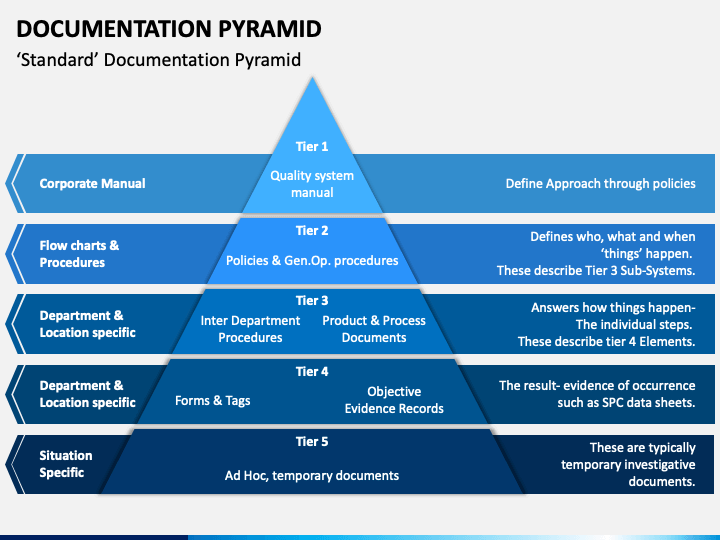
Quality Management System Pyramid
The QMS documentation is defined by each company based on regulatory and customer requirements. This article will discuss QMS documentation, its structure, requirements, benefits of well-documented and implemented documentation, and maintenance. It also explains the role of QMS software in managing QMS documentation effectively.