
Uzgodnij współczynniki w następujących reakcjach Cr2O3 + KNO3 + KOH K2CrO4 + KNO2 + H2O
Detailed information about the equation. Reaction conditions when applied H2S + H2SO4 + K2Cr2O7. Reaction process H2S + H2SO4 + K2Cr2O7. The result of the reaction H2S + H2SO4 + K2Cr2O7.

1.Oblicz stopnie utlenienia pierwiastków w następujących związkach SiO2, MgO, H2S, NH3, H3PO4
Solved and balanced chemical equation K2Cr2O7 + 3 H2S + 4 H2SO4 → K2SO4 + Cr2(SO4)3 + 3 S + 7 H2O with completed products. Application for completing products and balancing equations.. [Note: Sense of smell becomes rapidly fatigued & can NOT be relied upon to warn of the continuous presence of H2S. Shipped as a liquefied compressed gas.]

K2cr2o7 K2so3 H2so4 Cr2 So4 3 K2so4 H2o Margaret Wiegel
Detailed information about the equation. Reaction conditions when applied H2SO4 + K2Cr2O7 + K2S. Reaction process H2SO4 + K2Cr2O7 + K2S. The result of the reaction H2SO4 + K2Cr2O7 + K2S.

Используя метод инноэлектронного баланса,расставьте коэф. в окислитвосст. реакциях.Определите
Balance the chemical equation k2cr2o7+h2so4+so2=k2so4+cr2(so4)3+H2O Balance the chemical reaction K2Cr2O7+H2SO4+SO2=K2SO4+Cr2(SO4)3+H2O Potassium dicro.

Найдите сумму коэффициентов в правой части уравнения. H2S + H2SO4 + K2Cr2O7 = Cr2(SO4)3 + S
H2SO4 + K2CrO4 → H2O + K2Cr2O7 + K2SO4 | Equation Balance. Color some common substances.

how to balance h2o4 + h3s + 1 + h2o
Solved and balanced chemical equation K2Cr2O7 + 3 H2O2 + 4 H2SO4 → Cr2(SO4)3 + 7 H2O + K2SO4 + 3 O2 with completed products. Application for completing products and balancing equations. Chemical Equations online! Submit. Advanced search. K 2 Cr 2 O 7 + 3 H 2 O 2 + 4 H 2 S O 4 → Cr 2 (S O 4) 3 + 7 H 2 O + K 2 S O 4 + 3 O 2. This.

Action Of Acidified K2Cr2O7 On Hydrogen Sulphide D and F Block Elements Chemistry Class 12
Step 4: Substitute Coefficients and Verify Result. Count the number of atoms of each element on each side of the equation and verify that all elements and electrons (if there are charges/ions) are balanced. 0 K2Cr2O7 + 3 H2S + H2SO4 = 0 Cr2(SO4)3 + 0 K2SO4 + 4 S + 4 H2O. Reactants. Products.
Al + KMnO4 + H2SO4 = KHSO4 + Al2(SO4)3 + MnSO4 + H2O KNO3 + FeSO4 + H2SO4 = KHSO4 + Fe2(SO4)3
Click here:point_up_2:to get an answer to your question :writing_hand:balance the following equation by oxidation number methodk2cr2o7feso4h2so4rightarrow.

Napisz reakcje redoks, wskaż reduktor, utleniacz i zapisz reakcje połówkowe K2Cr2O7 + H2S
Step 4: Substitute Coefficients and Verify Result. Count the number of atoms of each element on each side of the equation and verify that all elements and electrons (if there are charges/ions) are balanced. 4 K2Cr2O7 + -3 H2S + 15 H2SO4 = 4 Cr2(SO4)3 + -8 S + 8 KHSO4 + 8 H2O. Reactants. Products.

please try to balance this equation K2Cr2O7 + H2SO4 > K2SO4 + Cr2(SO4)3 + H2O + O2 Brainly.in
K2Cr2O7+H2SO4+SO2=K2SO4+Cr2(SO4)3+H2O balance the redox reaction by ion electron method or half reaction method. k2cr2o7+h2so4+so2=k2so4+cr2(so4)3+h2o.

K2Cr2O7 + H2S + H2SO4 =S +K2SO4 + Cr2(SO4)3 + H2O is a very common reaction. The chemistry
Balance Chemical Equation - Online Balancer. Word equation; 4 Potassium dichromate + 3 Sulfane + 13 Sulfuric acid = 4 Potassium sulfate + 4 Chromium(III) sulfate + 16 Water

Презентация по Химии "Хром" скачать смотреть бесплатно
Here's what I got. Start by writing the unbalanced chemical equation. Potassium dichromate, "K"_2"Cr"_2"O"_7, will react with hydrosulfuric acid, which is aqueous.

Ki reacts with h2so4 producing i2 and h2s the volume of 0.2 N h2so4 r askIITians
Step 4: Substitute Coefficients and Verify Result. Count the number of atoms of each element on each side of the equation and verify that all elements and electrons (if there are charges/ions) are balanced. K2Cr2O7 + 4 H2So4 + 3 H2S = K2So4 + Cr2(So4)3 + 7 H2O + 3 S. Reactants.
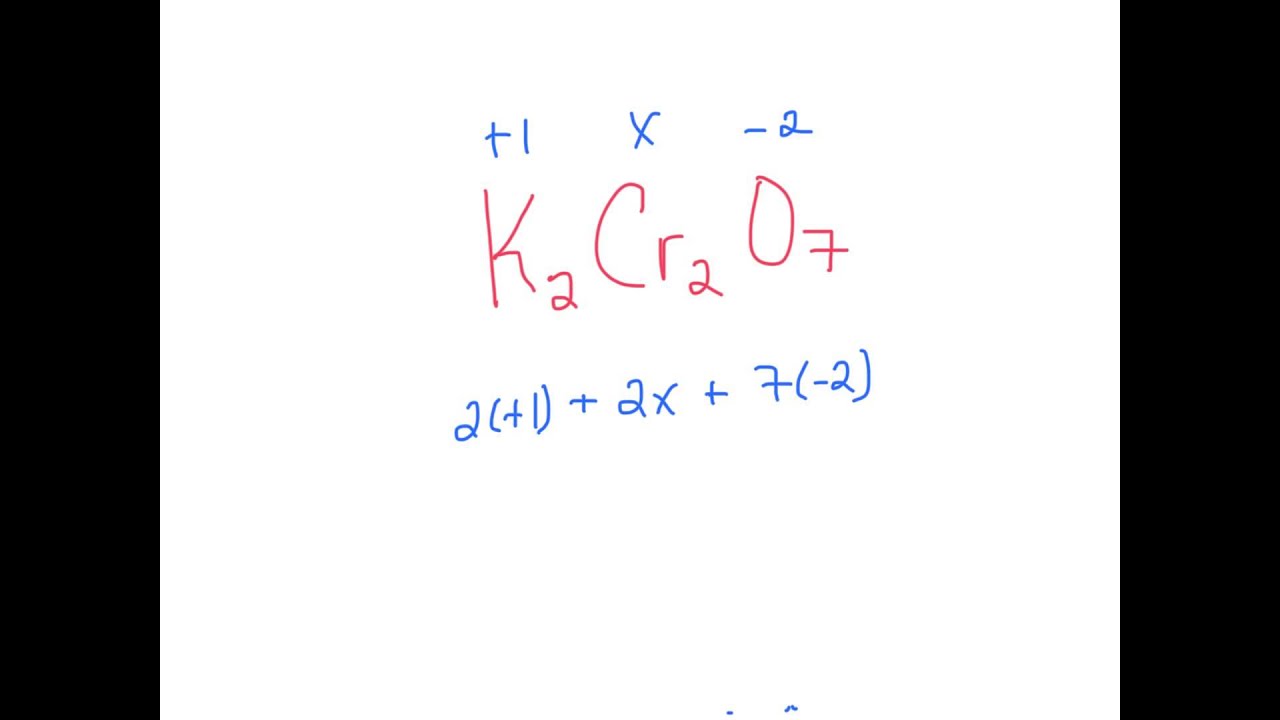
K2Cr2O7 YouTube
The hydrogen atoms are already balanced, so we don't need to make any changes. The equation now looks like this: K2Cr2O7 + H2SO4 ---> K2SO4 + Cr2(SO4)3 + H2O + 2O2 Answer 3. Finally, we need to balance the number of potassium and sulfate ions. On the left side, we have 2 potassium ions from K2Cr2O7 and 1 sulfate ion from H2SO4.

K2Cr2O7 + H2So4 / Which compound can be most easily oxidized... Clutch Prep It must have
About K2Cr2O7 H2S H2SO4. Potassium dichromate (K2Cr2O7) is a common chemical inorganic reagent most commonly used as an oxidizing agent in various laboratories. It's a crystalline ionic strong, very bright, red-orange. Among chemists, Potassium Dichromate is very common in determining the unknown concentration of secondary standard substances solution.

a beakle filled with liquid and flasks containing orange, red and blue liquids
Step 4: Substitute Coefficients and Verify Result. Count the number of atoms of each element on each side of the equation and verify that all elements and electrons (if there are charges/ions) are balanced. 4 K2Cr2O7 + 3 H2S + 13 H2SO4 = 4 K2SO4 + 4 Cr2(SO4)3 + 16 H2O. Reactants.