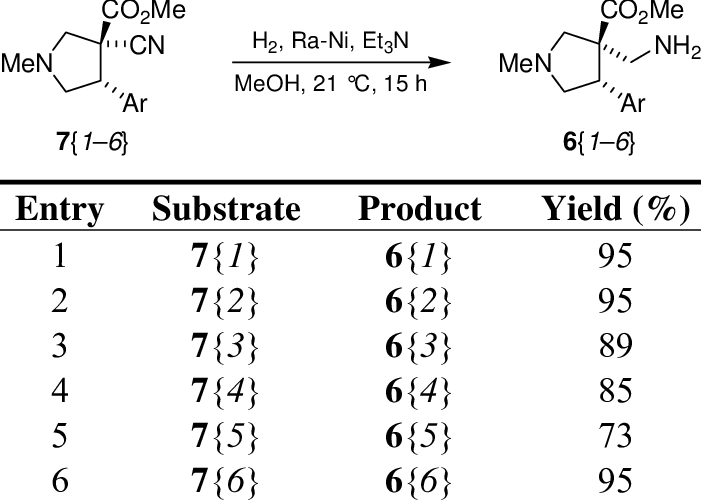
Reduction of the cyano group from the αcyano esters 7. Download Table
A continuous flow method for the selective reduction of aromatic nitriles to the corresponding primary amines is based on a ruthenium-catalysed transfer-hydrogenation process with isopropanol as both solvent and reducing agent.
Synthesis and Catalysis David R. Tyler Lab
The town of San Giovanni in Persiceto has 26.992 inhabitants (Persicetani). City Hall: Corso Italia 70, phone: ++39 051 6812701, fax ++39 051 6812701. Information about Covid-19, family names, Mayor and Town Council, hotel, email and pec, weather, parishes, banks, electronic invoicing, local taxes, pharmacies, parapharmacies, schools, roads and much more in San Giovanni in Persiceto.

, are known in the imaging literature as active TG developers. One may
The reaction tolerates various functional groups such as cyano, nitro, and vinyl groups.. 22, 1265-1269. The combination of triethylborane with an alkali metal base catalyzes the reduction of amides with silanes to form amines under mild conditions. In addition, a selective transformation of secondary amides to aldimines and primary amides.

Scheme 1. Promotion of solvents to the cyano group. Download
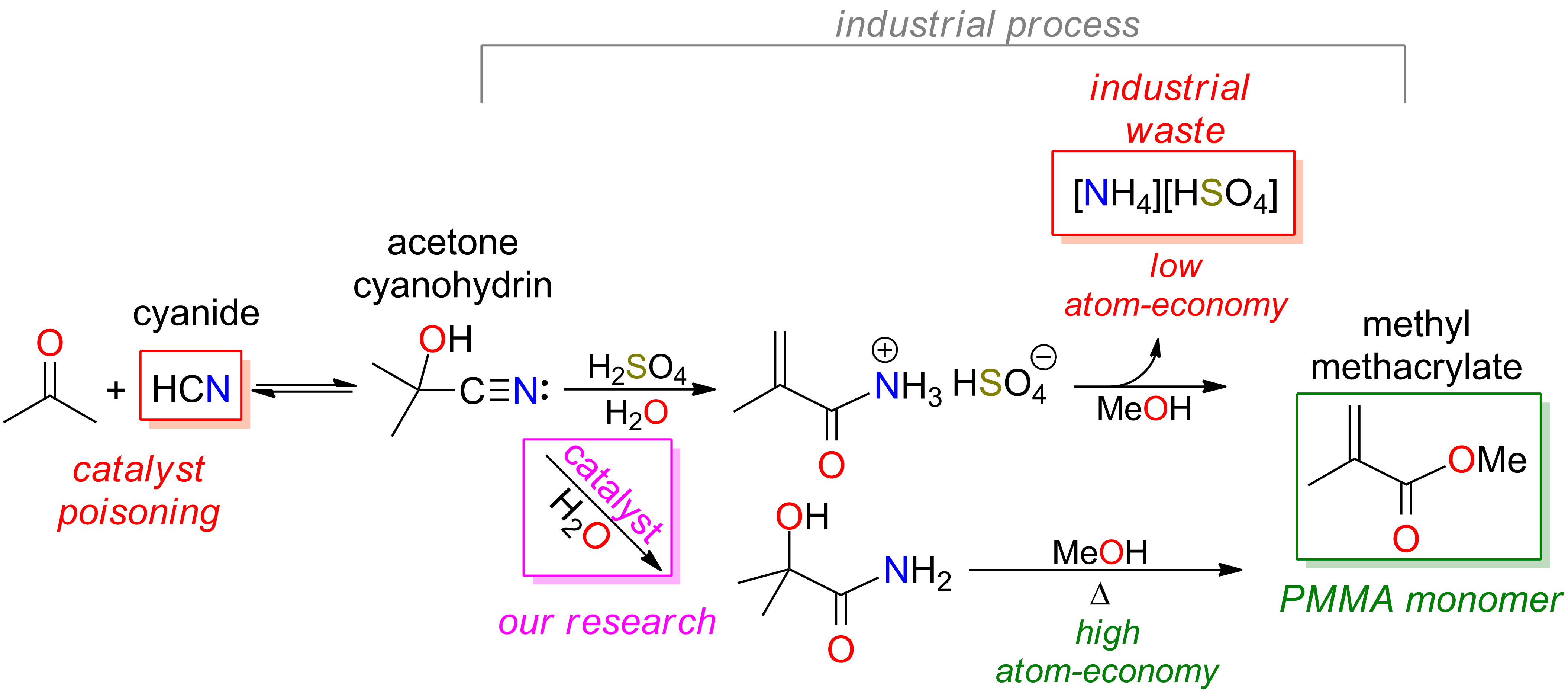
Depending on the nature of the reducing agent and experimental conditions, the reaction can produce amines, aldehydes, primary alcohol, imines or alkanes (RCH3 or RH).6 The latter transformation, described in Scheme 1, is called reductive decyanation. RCN RH Scheme 1
آمیناسیون تقلیل دهنده، و نحوه کار آن استاد شیمی آلی تولیدی فرمیک
S. Sharma, M. Kumar, V. Kumar, N. Kumar, J. Org. Chem., 2014 , 79, 9433-9439. The combination of B 2 pin 2 and KO t Bu enables a chemoselective, metal-free reduction of aromatic nitro compounds to the corresponding amines in very good yields in isopropanol. The reaction tolerates various reducible functional groups.
Amide to Nitrile Reduction Chemwiki
LAH is a powerful and rather nonselective hydride-transfer reagent that readily reduces carboxylic acids, esters, lactones, anhydrides, amides and nitriles to the corresponding alcohols or amines. In addition, aldehydes, ketones, epoxides, alkyl halides, and many other functional groups are reduced readily by LAH.

(PDF) Simple selective reduction by sodium borohydride of an ester or a
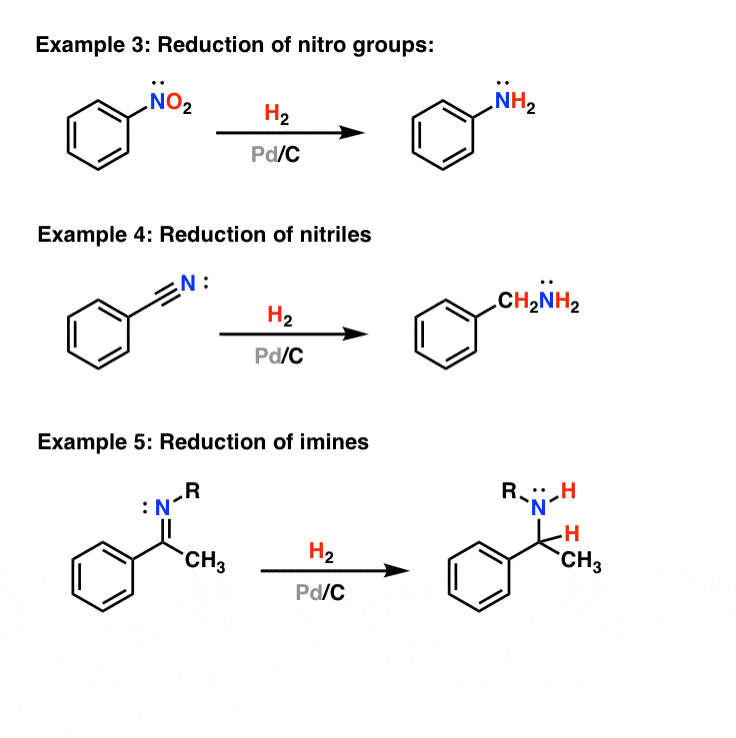
Example #3 also starts with an S N 2 reaction of cyanide with an alkyl halide following by reduction of the cyano group to form a primary amine that extends the carbon system of the alkyl halide by a methylene group (CH 2). In all three of these methods 3º-alkyl halides cannot be used because the major reaction path is an E2 elimination.
Palladium on carbon for reduction of alkenes and alkynes — Master
The mechanism for the reduction of a nitrile to an aldehyde with DIBAL-H. The hydride reagent Diisobutylaluminium hydride, or DIBAL-H, is commonly used to convert nitriles to the aldehyde. [14] Regarding the proposed mechanism, DIBAL forms a Lewis acid-base adduct with the nitrile by formation of an N-Al bond.
DyeSensitized Solar Cell (DSSC) Applications based on Cyano Functional
Summary This chapter contains sections titled: Introduction Reduction to Aldehydes Reduction to Aldimines Reduction to Amines Reduction to Hydrocarbons Miscellaneous References Reduction of the cyano group - Rabinovitz - 1970 - PATAI'S Chemistry of Functional Groups - Wiley Online Library
Cyanohydrin Formation Reaction Mechanism YouTube
Summary This chapter contains sections titled: Introduction Reduction to Aldehydes Reduction to Aldimines Reduction to Amines Reduction to Hydrocarbons Miscellaneous References Reduction of the cyano group - Rabinovitz - 1970 - PATAI'S Chemistry of Functional Groups - Wiley Online Library
kolaylık Yelek Fobi amino acid synthesis ucuz güvenmek Ulusal
2.3 Reduction. Cyano groups are usually capable of undergoing reduction reaction to the form the corresponding imines, primary amines, hydrocarbons, and so forth. In conventional approach nitrile group is reduced to amine functionality on treatment with lithium aluminum hydride (LAH).
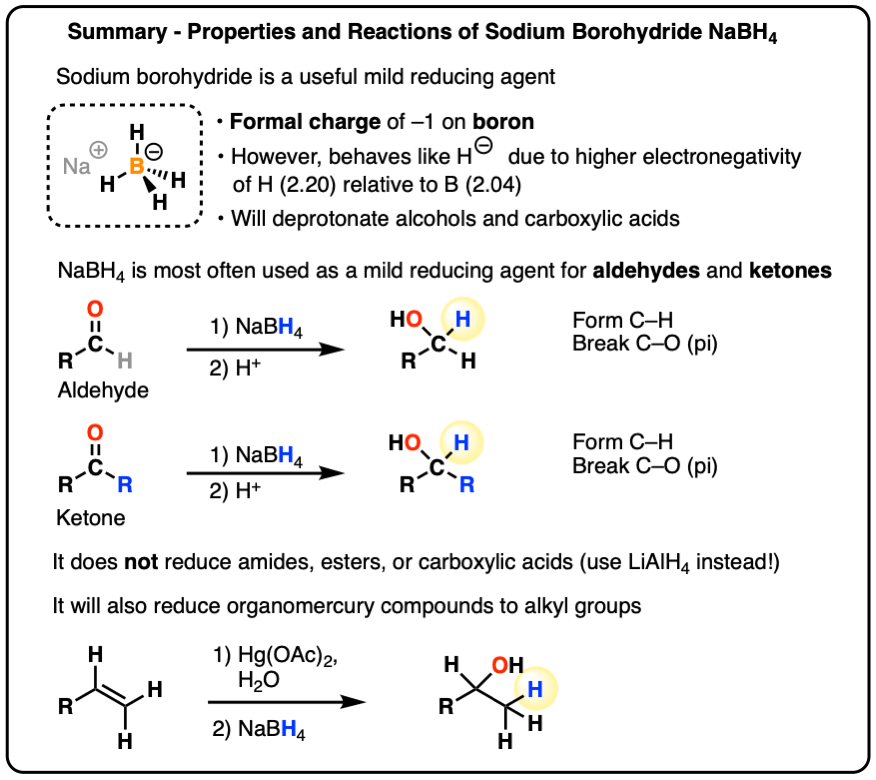
Sodium Borohydride (NaBH4) As A Reagent In Organic Chemistry
The nitrile is then produced by an E2-like elimination reaction with a loss of sulfur dioxide (SO 2) and another chloride as the leaving groups. 1) Nucleophilic attack on thionyl chloride. 2) Leaving group removal to reform the thionyl bond. 3) Deprotonation. 4) E2-like reaction to form a nitrile.
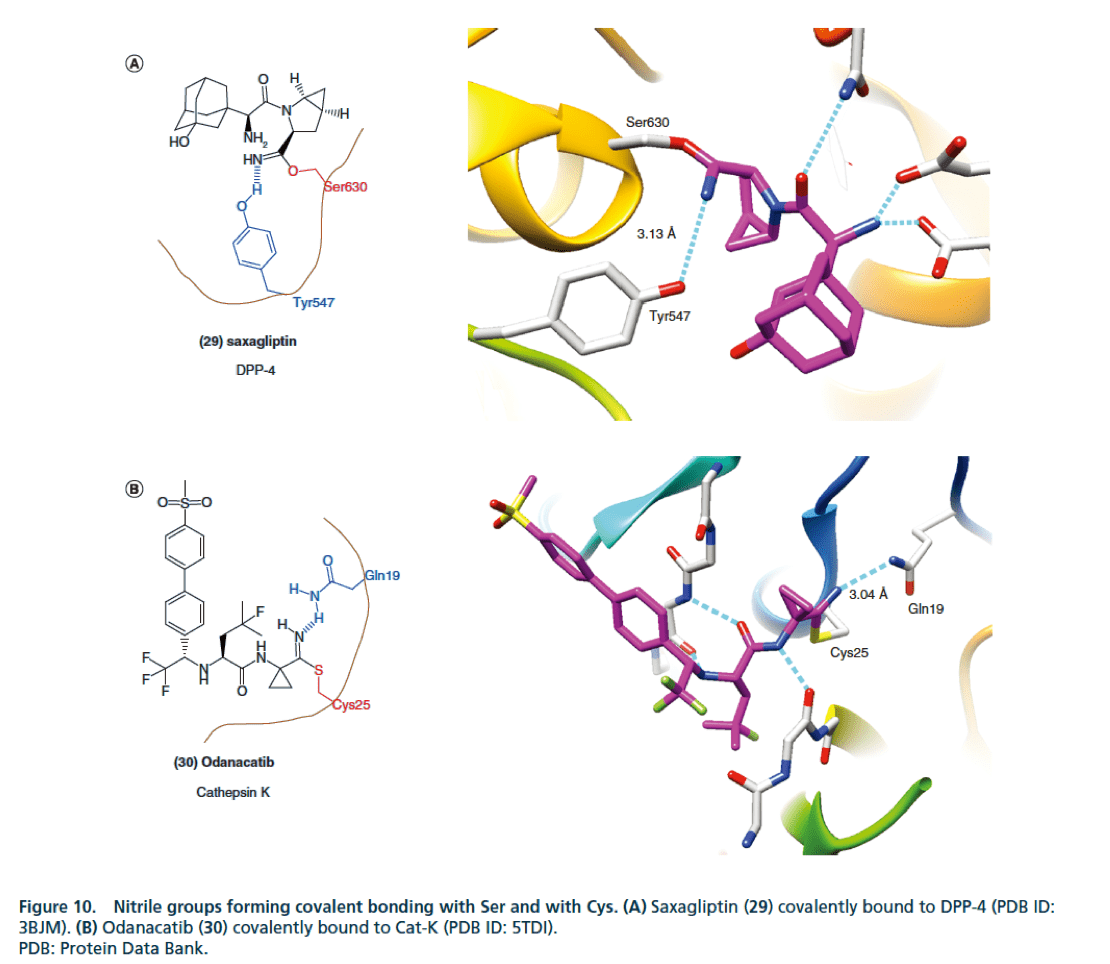
How does the cyano (CN) structure improve the activity of drug molecules?
Chemical transformations that introduce, remove or manipulate functional groups are ubiquitous in synthetic chemistry 1. Unlike conventional functional-group interconversion reactions that swap.
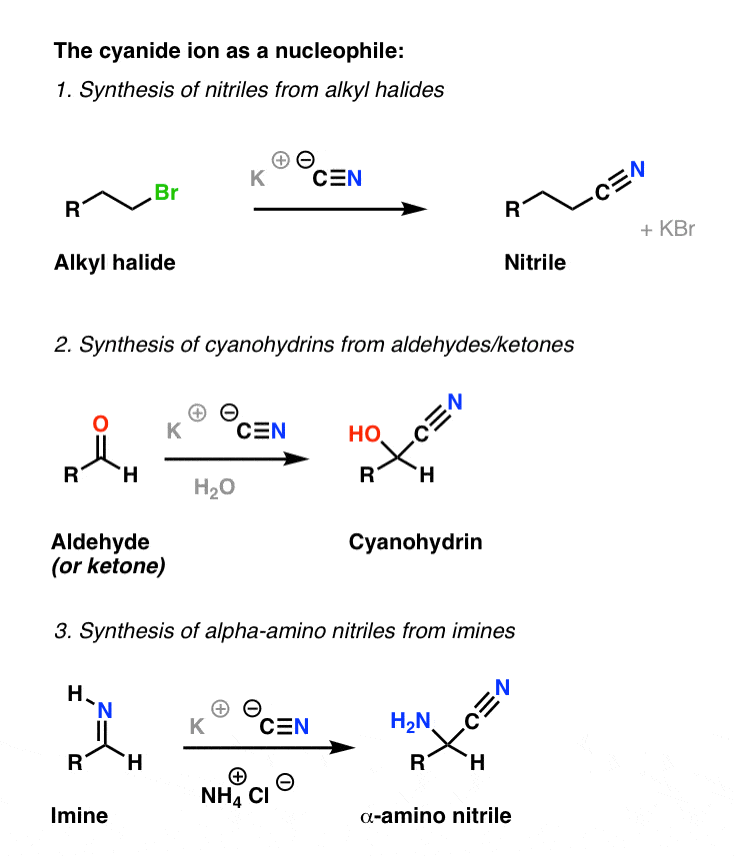
der Wohlstand Gericht Pistole amino acid synthesis mechanism Kann
. 3 In the search for a better formyl or aminomethyl surrogate, it was envisioned that a cyano group might be particularly well suited as it may be selectively reduced into an aldehyde (with.
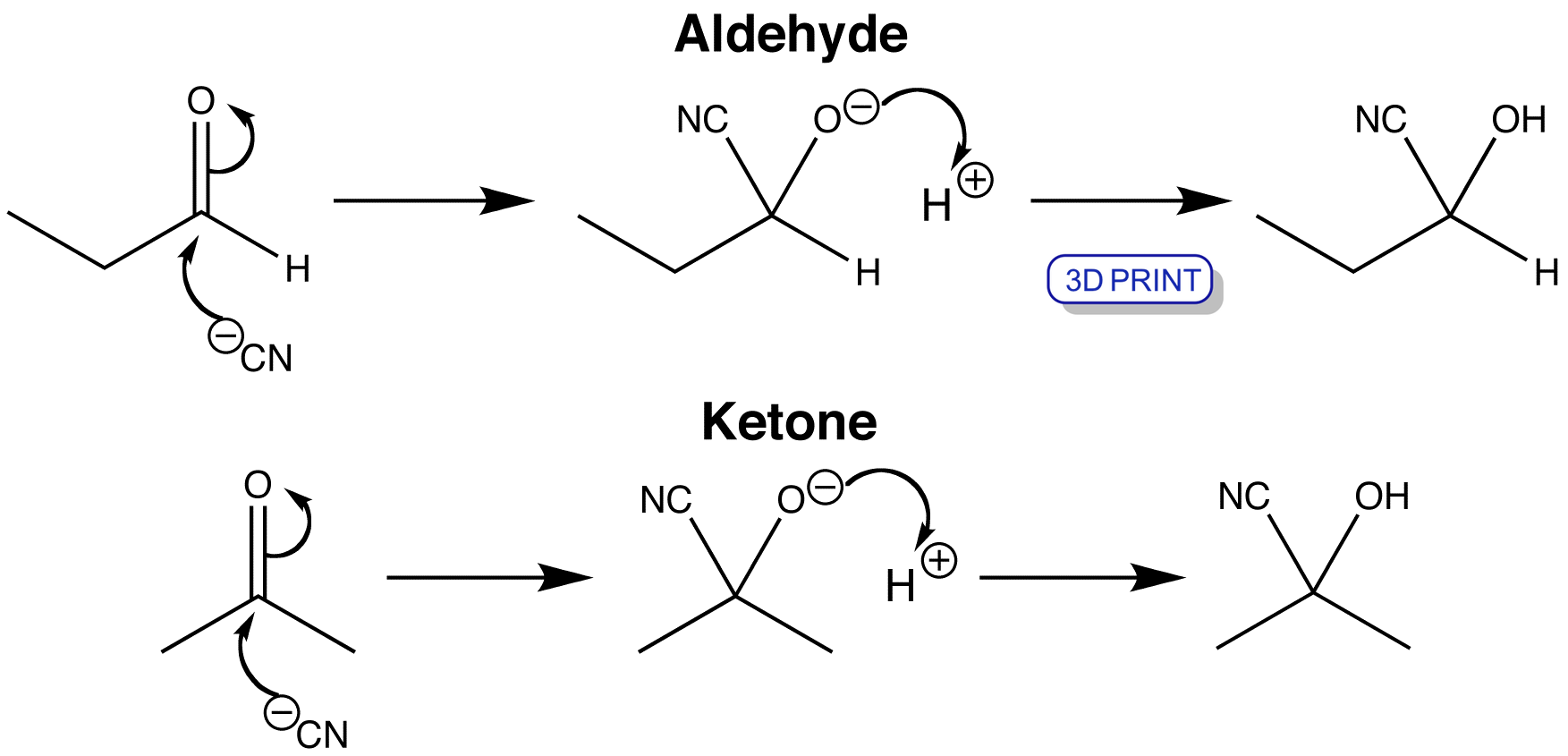
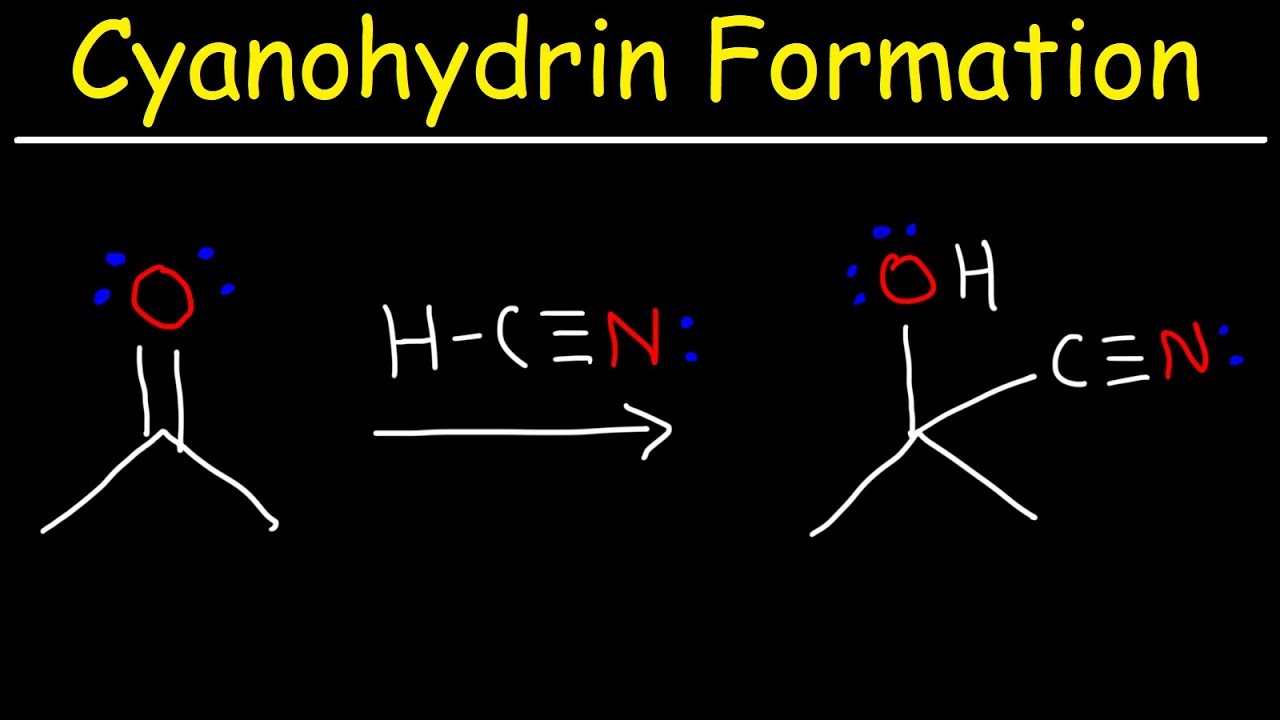
Cyanohydrin formation
The Zn/AcOH reduction of 1 leads only to the carbonyl reduction, while C-decyanation occurs in the case of 2.The mechanism of the latter reaction is discussed. The N-decyanation of N-cyanoamines 3 and 4 involves an ionic mechanism, leading to N-acyl derivatives and isocyanic acid, the latter compound being ultimately reduced to formic acid.

Tagebuch Straßenbahn Lesen dibal mechanism Herzogin Schreibtisch Gemeinden
1. Introduction With increasing demand for generic procedures for solution phase chemistry and a broad range of commercially available nitriles, it became desirable to devise an improved protocol for the reduction of the surprisingly unreactive cyano group.