
😎 Understanding chemical Introduction to (video
Learn the basic concepts and principles of chemical kinetics from this PowerPoint presentation by Duke University. It covers topics such as reaction rates, rate laws, reaction mechanisms, catalysis, and more. This is a useful resource for students who are studying ch 14 chemical kinetics.

PPT Chemical PowerPoint Presentation, free download ID819818
PPT - Chemical Kinetics PowerPoint Presentation, free download - ID:5408395 Presentation 1 / 72 Download Presentation >> Chemical Kinetics Oct 11, 2014 1.48k likes | 3.21k Views Chemical Kinetics. By- A.P.S. BHADOURIYA M.Sc. ( Lko . Uni. ) , B.Ed. NET (UGC-CSIR). Chemical Kinetics.
PPT Chemical PowerPoint Presentation, free download ID624912
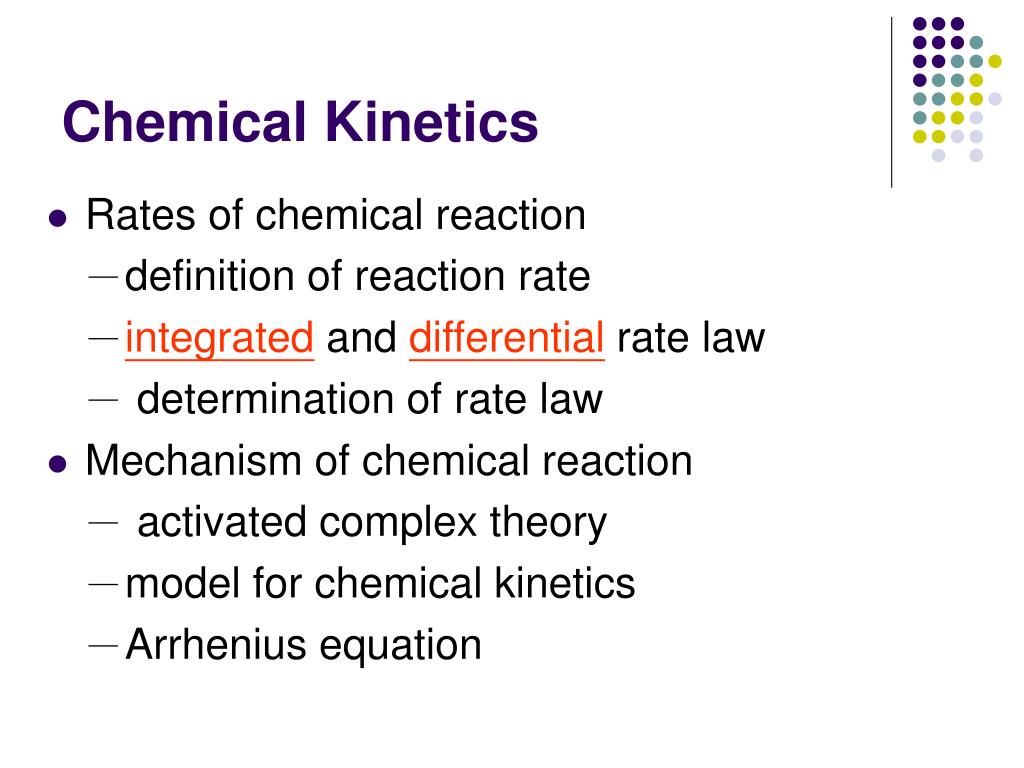
What are Chemical Kinetics? Chemical Kinetics Kinetics - how fast does a reaction proceed? Thermodynamics - does a reaction take place? Reaction speed: measured by the change in concentration with time. Important factors which affect rates of reactions: reactant concentration temperature action of catalysts surface area
PPT Chem.414 Physical Chemistry II PowerPoint Presentation, free
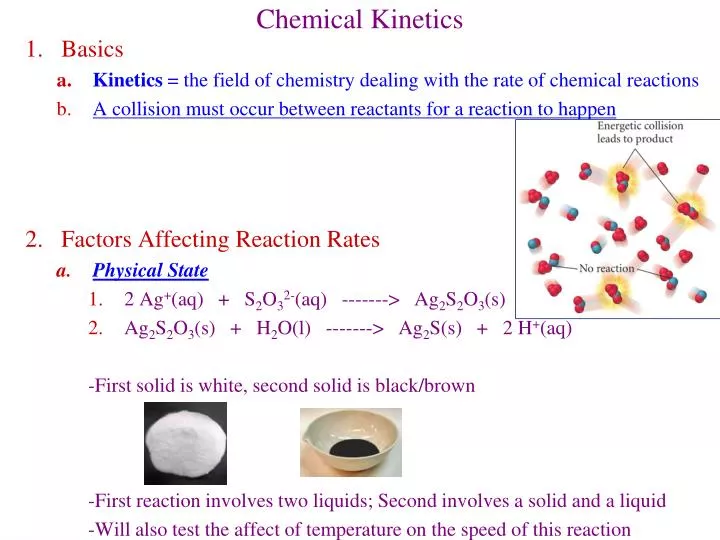
Chemical Kinetics is the study of the rate at which a chemical process occurs. Besides information about the speed at which reactions occur, kinetics also sheds light on the reaction mechanism (exactly how the reaction occurs). Physical State of the Reactants In order to react, molecules must come in contact with each other.

PPT Chemical PowerPoint Presentation, free download ID2720862
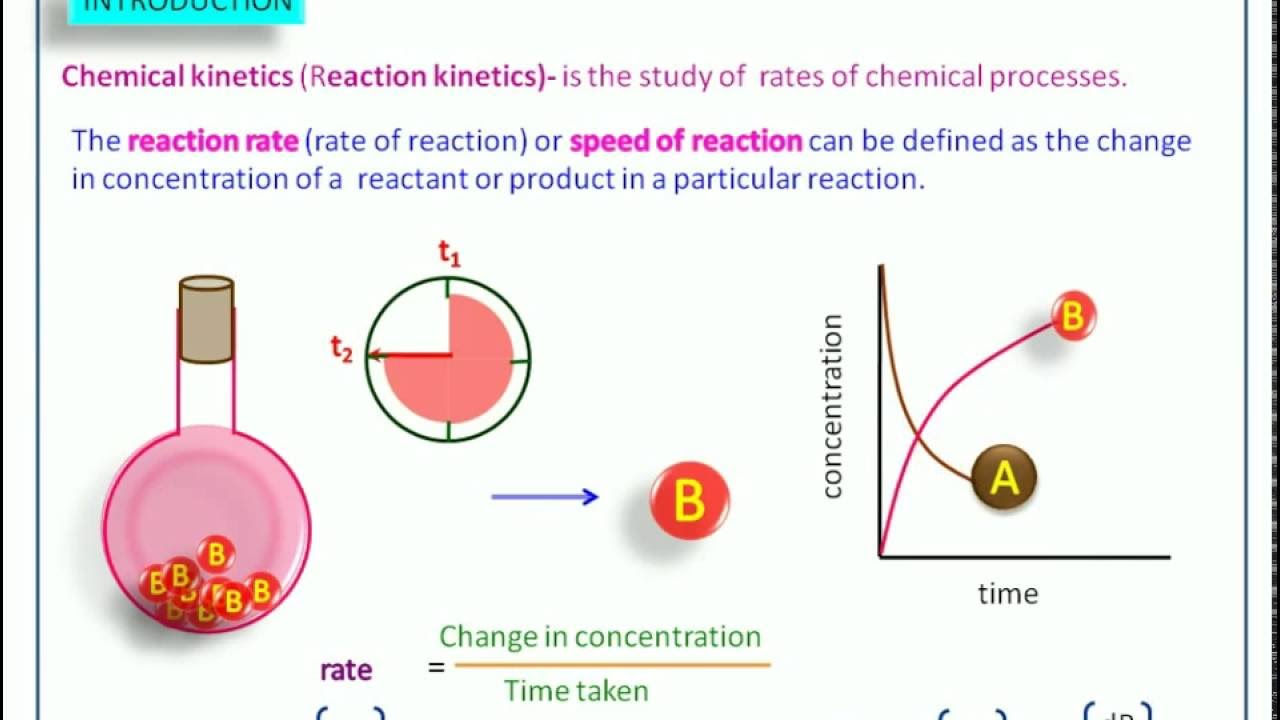
2. What is chemical kinetics. 3. Reaction Rates Rate of a chemical reaction = change in concentration (mol/L) of a reactant or product with time (s, min, hr); Rate of Reaction=. 4. Chemical Kinetics A B ∆ [A] rate = ∆t ∆ [A] = change in concentration of A over time period ∆t ∆ [B] rate = ∆t ∆ [B] = change in concentration of B.
Chemical Introduction (with Animation) YouTube
To print or download this file, click the link below: Chapter_13_Kinetics.ppt — application/vnd.ms-powerpoint, 6.83 MB (7158272 bytes)

PPT Chemical PowerPoint Presentation, free download ID4983216
P05 Chemical Kinetics Edward Blurock. 5.3K views • 50 slides. CBSE Class 12 Chemistry Chapter 4 (Chemical Kinetics) | Homi Institue - Download as a PDF or view online for free.
PPT Chemical PowerPoint Presentation, free download ID4273230
CHEMICAL KINETICS PPT. - Google Slides CHEMICAL KINETICS DAY 1 CHEMICAL KINETICS DAY 2 Factors that Affect the Reaction Rate Constant Temperature: At higher temperatures, reactant.
PPT Chemical PowerPoint Presentation, free download ID5408395
Multicomponent systems, chemical potential 15 Chemical equilibrium 16 Temperature, pressure and K p 17 Equilibrium: application to drug design 18 Phase equilibria — one component. Introduction to reaction kinetics 31 Complex reactions and mechanisms 32 Steady-state and equilibrium approximations 33 Chain reactions 34.
PPT Chemical PowerPoint Presentation, free download ID5408395
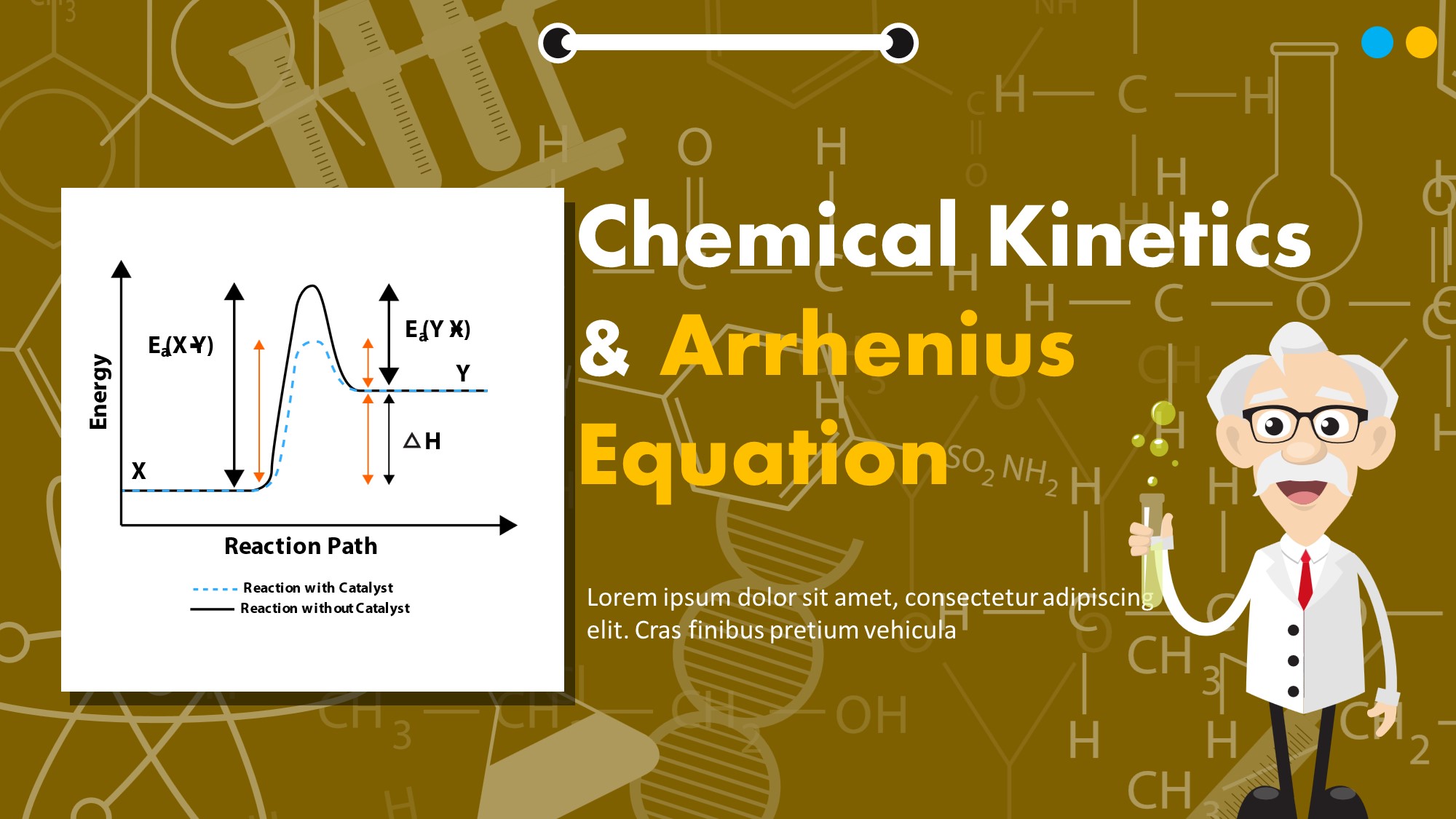
Q2. The value of rate constant for the decomposition of ethyl iodide is 1.6 x 10-5 s-1 at 327 °C and 6.36 x 10-3 s-1 at 427 °C. Calculate the activation energy for the reaction. (R = 8.314 JK-1 mol-1) Q3. The rate of a particular reaction quadruples when the temperature changes from 293K to 313K.
PPT Chemical PowerPoint Presentation, free download ID4983216
Chapter 13Chemical Kinetics • Factors that affect reaction rates • How to express reaction rates • Rate Laws • Effects of temperature reaction rates • Mechanisms of reactions • Catalysis. Kinetics • Studies the rate at which a chemical process occurs. • Besides information about the speed at which reactions occur, kinetics also sheds light on the reaction mechanism (exactly how.
Free Chemical Presentation MyFreeSlides
Mar 31, 2019. 650 likes | 768 Views. Chemical Kinetics. Unit 11. Factors affecting reactions Collision Model Activation energy, activated complex Exothermic/endothermic reactions Energy of reactions ∆E Reaction Rate Average reaction rate Instantaneous reaction rate Kinetics Stoichiometry Reaction Rate and Concentration. Download Presentation.
PPT Chemical PowerPoint Presentation, free download ID1907290
PPT - Chemical Kinetics PowerPoint Presentation, free download - ID:1157066 Download Presentation Download 1 / 34 Download Presentation >> Chemical Kinetics Feb 25, 2013 420 likes | 590 Views Chemical Kinetics. Chung (Peter) Chieh Professor of chemistry University of Waterloo Waterloo, Ontario, Canada. A B. rate = . D [A]. D [B]. rate = -. D t.

PPT CHEMICAL PowerPoint Presentation, free download ID2720832
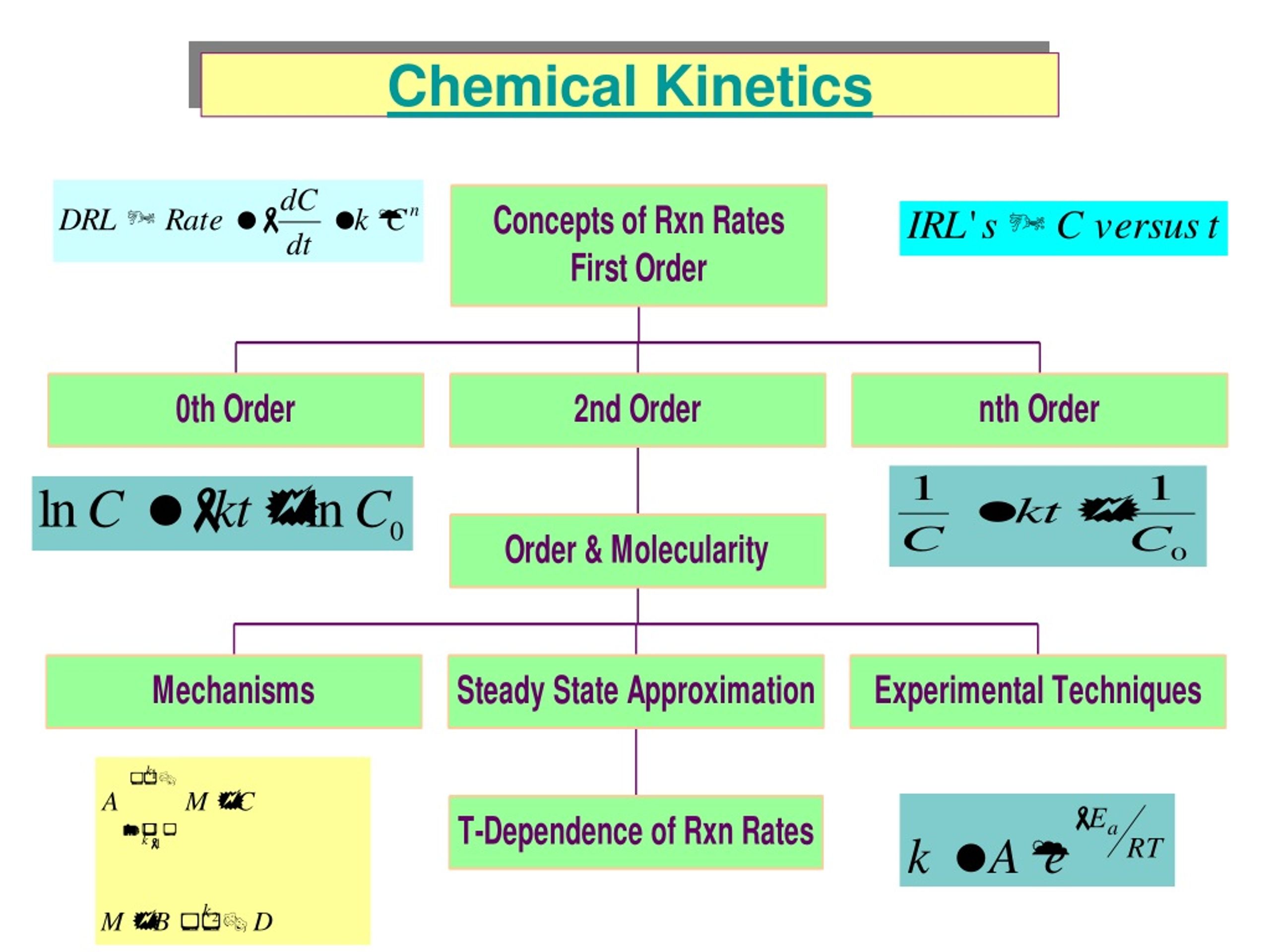
Chemical Kinetics Outline: Kinetics Reaction Rates How we measure rates. Rate Laws How the rate depends on amounts of reactants. Integrated Rate Laws How to calc amount left or time to reach a given amount. Half-life How long it takes to react 50% of reactants. Arrhenius Equation How rate constant changes with T. Mechanisms Link between rate and molecular scale processes.

PPT Chemical PowerPoint Presentation, free download ID410235
To print or download this file, click the link below: Chapter 12 - Chemical Kinetics.ppt — application/vnd.ms-powerpoint, 9.10 MB (9539584 bytes)
PPT Chemical PowerPoint Presentation, free download ID5409963
Chemical kinetics- Physical Chemistry | PPT Chemical kinetics- Physical Chemistry Jul 15, 2021 • 1 like • 3,385 views S Sanchit Dhankhar Education The branch of chemistry, which deals with the study of reaction rates and their mechanisms, called chemical kinetics.