
41 lewis dot diagram for c2h2 Diagram For You
C2H2 Molecular Geometry / Shape and Bond Angles (see description for note) Wayne Breslyn 725K subscribers Join Subscribe Subscribed 395 Share Save 121K views 10 years ago A quick explanation of.
C2h2 Estructura De Lewis Blogan
The Lewis structure for C2H2, also known as ethyne or acetylene, is a diagram that shows the arrangement of valence electrons and the bonding between atoms in a molecule. This structure is essential in understanding the properties and behavior of C2H2 in chemical reactions.
Estrutura De Lewis C2h2 AskSchool
For C2H2 Lewis structure, we will first place both the Carbon atoms in the centre as it is less electronegative than the Hydrogen atoms. Here both the Carbon atoms take the central position, and the Hydrogen atoms are arranged around it. If you look at the Hydrogen atoms, it only needs one valence electron to attain a stable structure.

Which is the correct Lewis dot structure of C2H2
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".
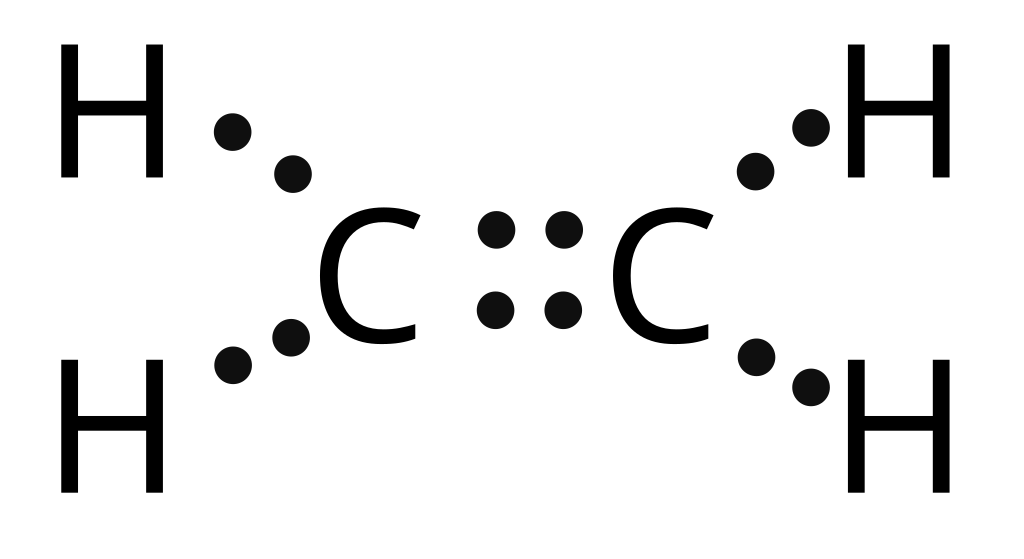
Lewis Dot Diagram For C2h2
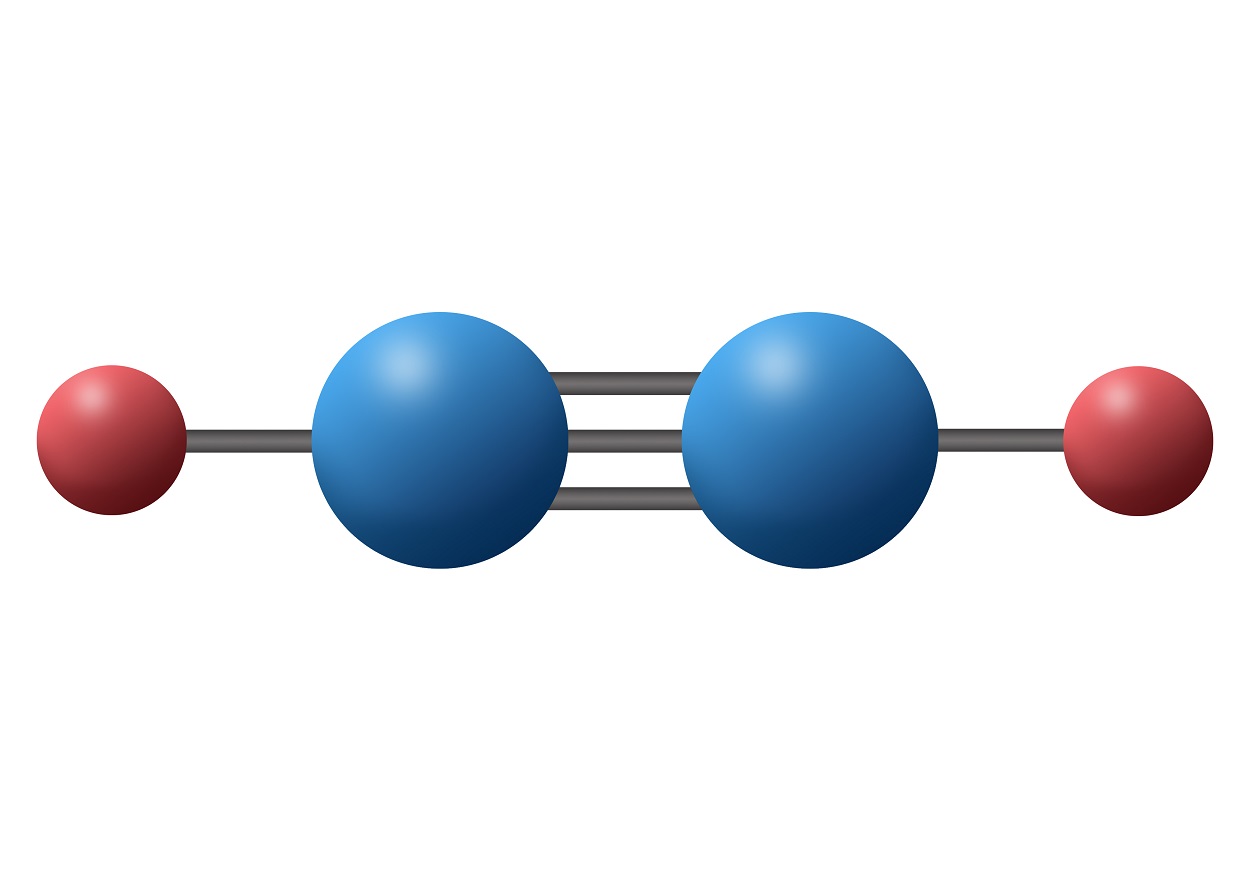
C 2 H 2 (acetylene or ethyne) contains two carbon atoms and two hydrogen atoms. There is a triple bond between carbon atoms and hydrogen atoms are joint with carbon atoms though sigma bonds. There are no lone pairs on carbon or hydrogen atoms. In this tutorial, we are going to learn how to draw the lewis structure of C 2 H 2 step by step.

Estructura De Lewis De C2h2 Blogan
By Jay Rana / Last Updated On: June 21, 2023 So you have seen the above image by now, right? Let me explain the above image in short. C2H2 lewis structure has a triple bond between the two Carbon atoms (C) and a single bond between the Carbon atom (C) and Hydrogen atom (H).

C2H2 Lewis structure ,Valence Electrons, Formal Charge
Lewis structure of C2H2 (or Acetylene or Ethyne) contains one triple bond between the two Carbon (C) atoms and two single bonds between Carbon (C) & Hydrogen (H) atoms. Let's draw and understand this lewis dot structure step by step. (Note: Take a pen and paper with you and try to draw this lewis structure along with me.

Lewis electrondot structure of C2H2 YouTube
In the C 2 H 2 Lewis structure, there is a triple bond between the two carbon atoms, and each carbon is attached with one hydrogen atom, and none of the atoms has a lone pair. Contents Steps #1 First draw a rough sketch #2 Mark lone pairs on the atoms #3 Calculate and mark formal charges on the atoms, if required
C2H2 Lewis structure ,Valence Electrons, Formal Charge
I quickly take you through how to draw the Lewis Structure of CHCH (Acetylene or ethyne). I also go over hybridization, shape, sigma, pi bonding and bond ang.

How do you draw the Lewis structure for C2H2? Ethyne or Acetylene
C2H2 is a linear molecule in form of geometry and the Lewis structure of C2H2 shows that the carbon atom has four valence electrons, while the hydrogen atom has one valence electron as it is an s-block element. Name of Molecule. Acetylene or ethyne. Chemical Formula. C2H2.

C2H2 lewis dot structure
Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.

Estrutura De Lewis Do C2H2 Detalhes científicos
C 2 H 2 is the chemical formula of the simplest and the first member of the alkyne family. It is most commonly known as acetylene in the scientific world. However, the IUPAC name for acetylene is ethyne. It exists as a colorless gas (molar mass = 26.04 g/mol) at room temperature and has a garlic-like odor.

C2H2 Molecular Geometry / Shape and Bond Angles YouTube
Contents show Lewis Structure of Acetylene (C2H2) Lewis Structure is the pictorial representation showing how the valence electrons are participating in bond formation. To study this, first, it is crucial to know the electronic configuration of the participating elements.

C2H2 Lewis Structure, Molecular Geometry, Hybridization, Polarity, and
27K views 1 year ago A step-by-step explanation of how to draw the C2H2 Lewis Dot Structure (Acetylene (Ethyne)). For the C2H2 structure use the periodic table to find the total number of.

C2H2 Lewis structure ,Valence Electrons, Formal Charge
A. Definition and concept The C2H2 Lewis structure refers to the arrangement of atoms and electrons in a molecule of ethyne (C2H2) using Lewis dot diagrams. This involves representing each atom using its chemical symbol and drawing dots around it to represent its valence electrons.

How to Draw the Lewis Dot Structure for C2H2 Acetylene (Ethyne) YouTube
In drawing the Lewis structure for C 2 H 2 (also called ethyne) you'll find that you don't have enough valence electrons available to satisfy the octet for each element (if you use only single bonds). The solution is to share three pairs of valence electrons and form a triple bond between the Carbon atoms in C 2 H 2 .