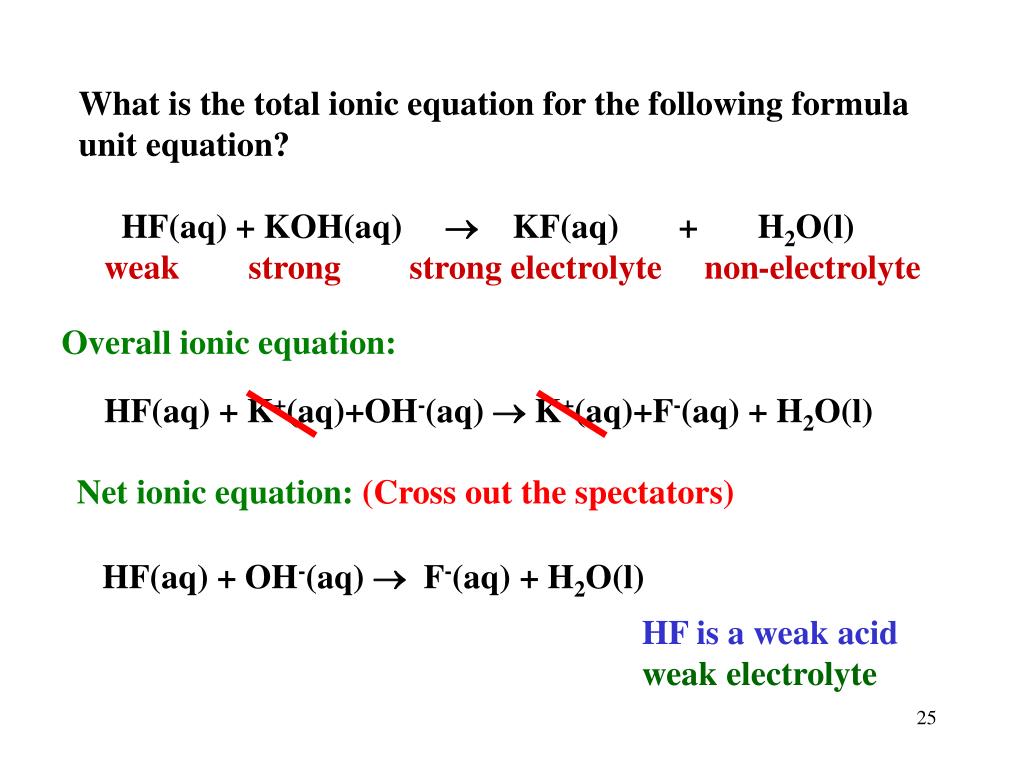
PPT Ionic Equations.... PowerPoint Presentation, free download ID6673081
To write the net ionic equation for Na2CO3 + HCl we follow three steps. First, we balance the molecular equation. Second, we break the soluble ionic compounds, the ones with an (aq) after.
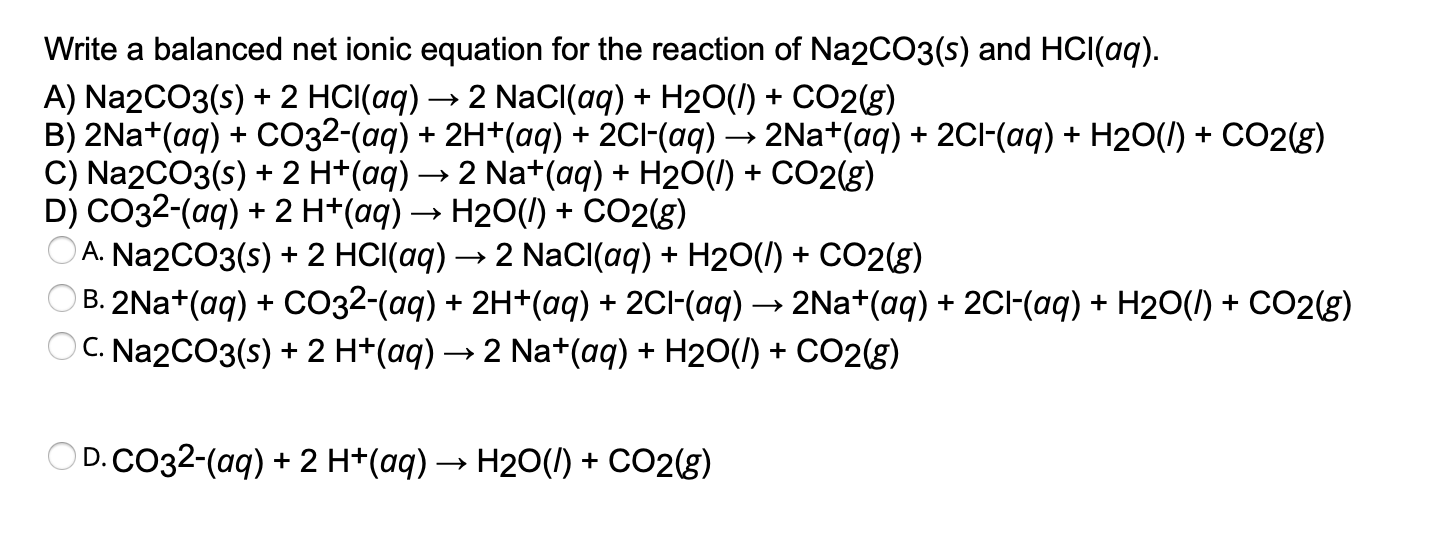
Solved Write a balanced net ionic equation for the reaction
Get the free "NET IONIC EQUATION CALCULATOR" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.

27) Give the net ionic equation for the reaction (if any) that Loccurs when aqueous solutions
One mole of Sodium Carbonate [Na 2 CO 3] and two moles of Hydrogen Chloride [HCl] react to form two moles of Sodium Chloride [NaCl], one mole of Water [H 2 O] and one mole of Carbon Dioxide [CO 2] Show Chemical Structure Image for HCl into tubes containing Na2CO3 → Cavitation caused by carbon dioxide (CO2) produced
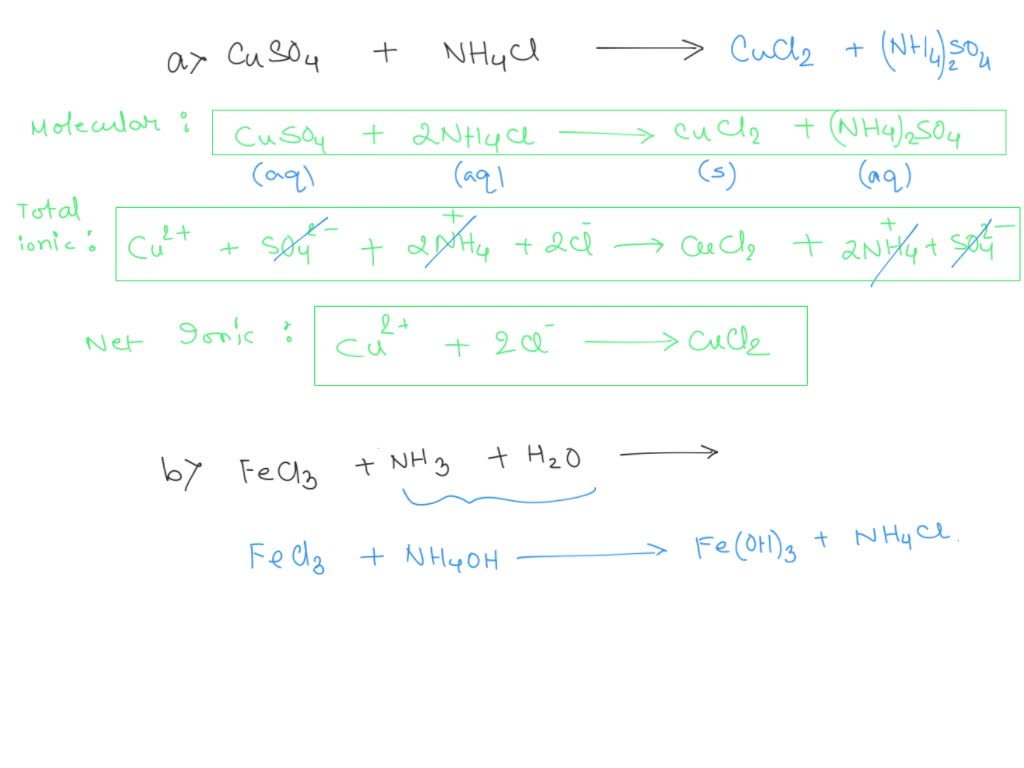
SOLVED write equations (molecular, total ionic, net ionic) for the reaction of CuSO4 and Na2CO3
Instructions Enter an equation of an ionic chemical equation and press the Balance button. The balanced equation will be calculated along with the solubility states, complete ionic equation, net ionic equation, spectator ions and precipitates. Use uppercase for the first character in the element and lowercase for the second character.

SOLVED Wrrite the net ionic equation for the following reaction Na2CO3 (aq)+ 2HCl (aq)→CO2 (g
Give the net ionic equation for the reaction (if any) that occurs when aqueous solutions of Na2CO3 and HCl are mixed. Group of answer choices 2 Na+ (aq) + CO32- (aq) + 2 H+ (aq) + 2 Cl- (aq) → H2CO3 (s) + 2 Na+ (aq) + 2 Cl- (aq) 2 H+ (aq) + CO32- (aq) → H2O (l) + CO2 (g) 2 Na+ (aq) + CO32- (aq) + 2 H+ (aq) + 2 Cl- (aq) → H2CO3 (s) + 2 NaCl (aq)
[Solved] Hydrochloric acid (HCl) reacts with sodium carbonate (Na2CO3),... Course Hero
Write a balanced net ionic equation for the reaction of Na2CO3(s) and HCl(aq). A) Na2CO3(s) + 2 HCl(aq) → 2 NaCl(aq) + H2O(1) + CO2(g) B) 2Na+(aq) + CO32-(aq) + 2H.
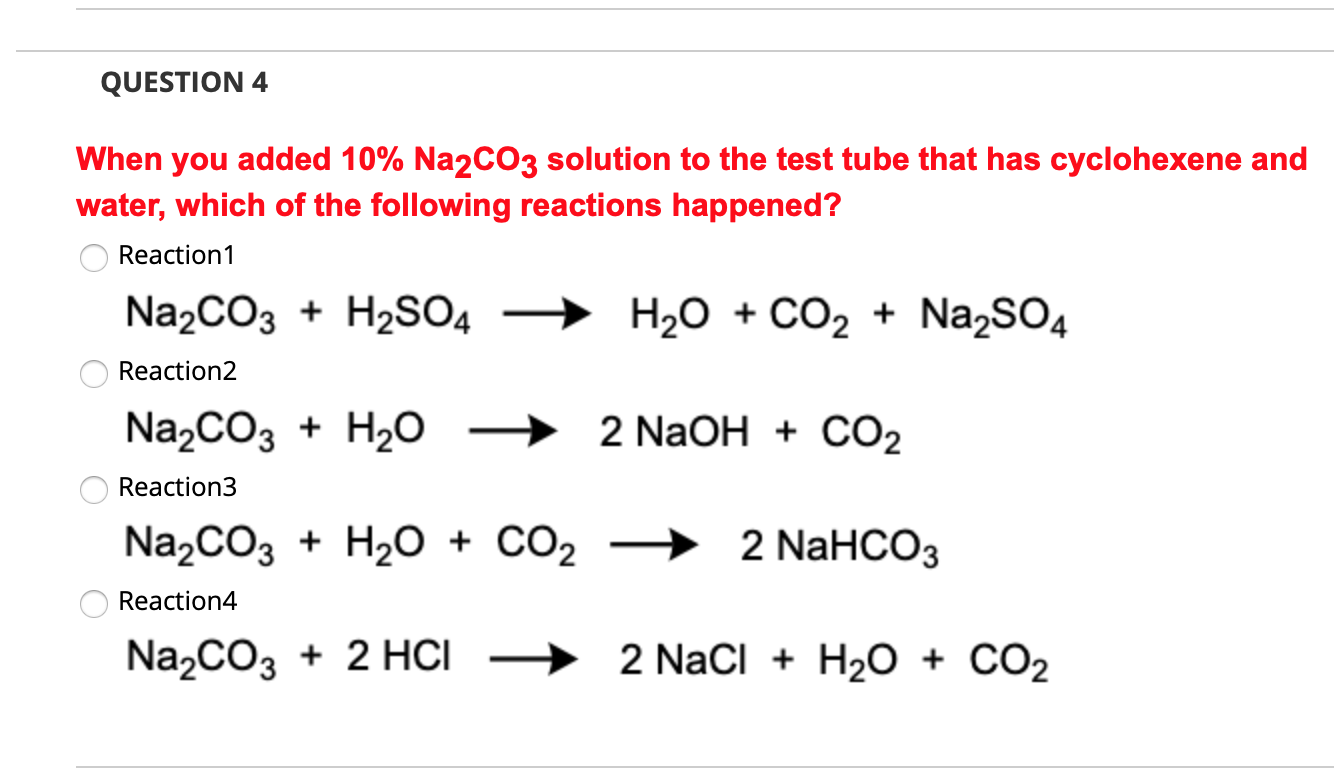
Solved QUESTION 4 When you added 10 Na2CO3 solution to the
The unbalance equation is: HCl + Na2CO3 = NaCl + H2O + CO2 Following steps are applied to equate the above-mentioned reaction: For the reaction to be balanced, the number of atoms of each element present on both the reactant and product sides must be equal. Table representing count of atoms from the unbalanced equation

Solved Experiment (?)B Standardization of HCl with Na2CO3
This chemistry video tutorial explains how to predict the products of the reaction between Sodium Carbonate and Hydrochloric Acid. It explains how to write.

Solved Experiment (?)B Standardization of HCl with Na2CO3
There are three main steps for writing the net ionic equation for NaHCO3 + HCl = NaCl + CO2 + H2O (Sodium bicarbonate + Hydrochloric acid). First, we balance.

Net Ionic Equation for BaCl2 + Na2CO3 = BaCO3 + NaCl YouTube
This is the equation given by my textbook for hydrolysis of sodium carbonate: NaX2COX3 +2HX2O HX2COX3 +2NaX+ +2OHX− N a X 2 C O X 3 + 2 H X 2 O H X 2 C O X 3 + 2 N a X + + 2 O H X −. and it mentions that sodium ion (NaX+) ( N a X +) does not tend to combine with the hydroxide ion (OHX−) ( O H X −) and I was wondering what prevents them.

How to Write the Net Ionic Equation for Na2CO3 + Sr(NO3)2 = NaNO3 + SrCO3 YouTube
HCl + Na2CO3 = NaCl + H2O + CO2 - Balanced Chemical Equation HCl + Na2CO3 = NaCl + H2O + CO2 - Balanced Chemical Equation Balanced Chemical Equation 2 HCl + Na 2 CO 3 → 2 NaCl + H 2 O + CO 2 ⬇ Scroll down to see reaction info and a step-by-step answer, or balance another equation. Reaction Information Word Equation

Balanced Equation of Sodium Carbonate and Hydrochloric Acid
This chemistry video explains how to write the balanced molecular equation and the net ionic equation of the reaction between Sodium Carbonate and Hydrochloric Acid. It also explains how to.

Net Ionic Equation and Complete Ionic Equation
Question: Write a balanced, net-ionic equation for the reaction of aqueous solutions of hydrochloric acid (HCI) and sodium carbonate (Na2CO3). a.

How to balance Na2CO3+HCl=NaCl+CO2+H2OChemical equation Na2CO3+HCl=NaCl+CO2+H2ONa2CO3+HCl
The balanced equation for the chemical reaction of Na₂CO₃ and HCl is given as: Na₂CO₃ (aq) + 2 HCl (aq) → 2 NaCl (aq) + CO₂ (g) + H₂O (l) The total ionic equation is the equation demonstrating the dissociation of the reactant and products into their respective ions. The total ionic equation for this reaction is:
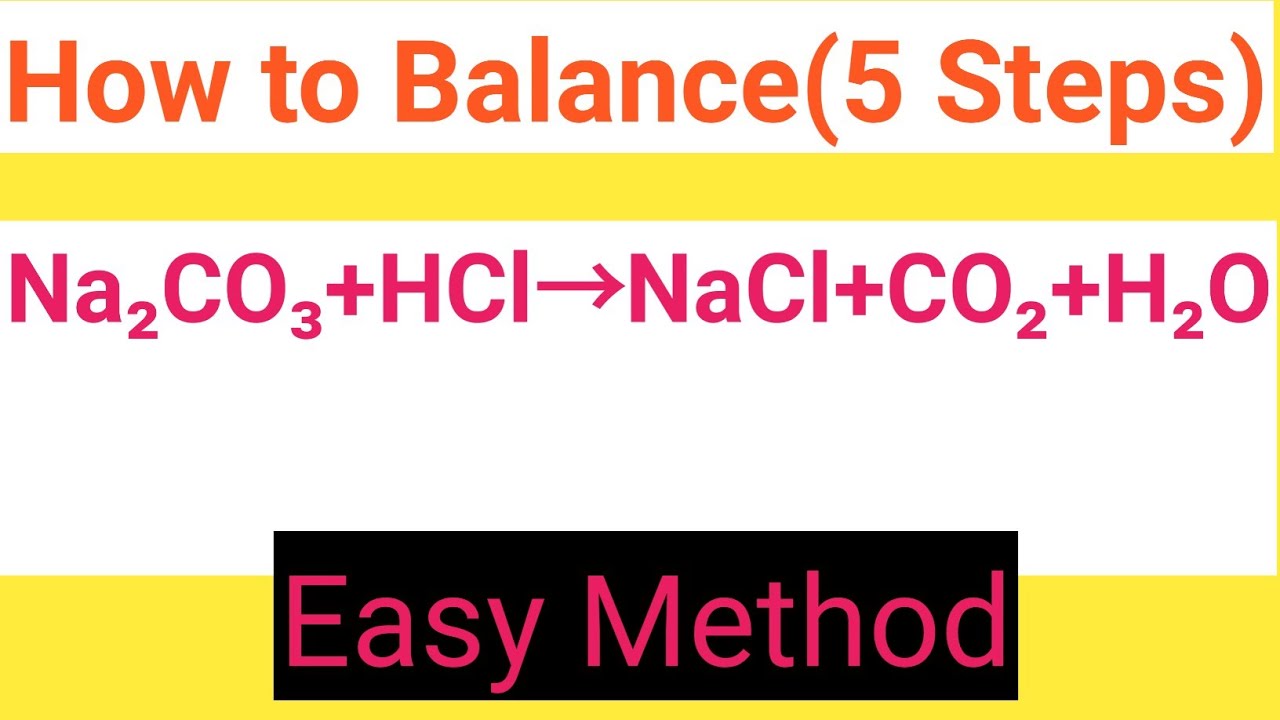
Na2CO3+HCl=NaCl+CO2+H2O Balanced EquationSodium carbonate+Hydrochloric acid=Sodium chloride
To write the net ionic equation for the reaction between Na2CO3 and HCl, we first need to write the balanced molecular equation: Na2CO3 + 2HCl -> 2NaCl + H2O + CO2. Next, we can identify the spectator ions , which are ions that do not participate in the chemical reaction.
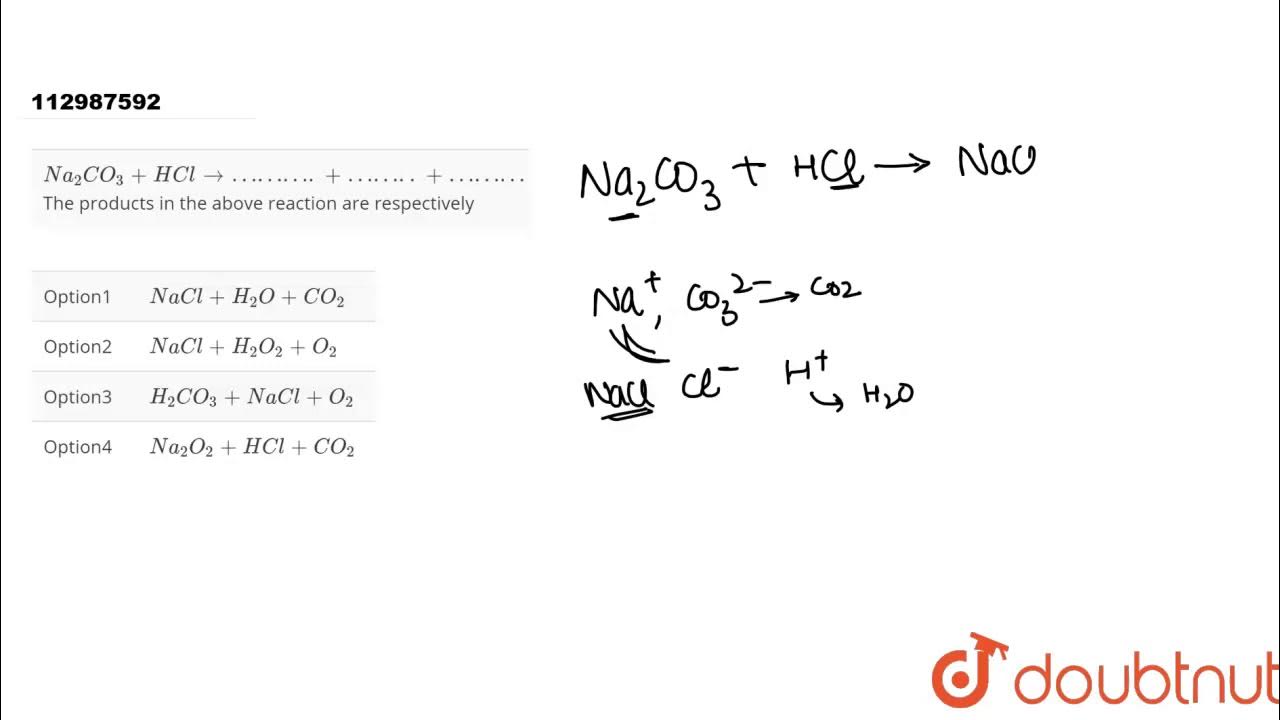
`Na_(2)CO_(3)+HCl to ………. + …….. + ………` The products in the above reaction are respectively
Sodium carbonate is a solid at room temperature. But it is a soluble inorganic compound in water and form a weak basic solution. Will sodium carbonate + hydrochloric acid give an acidic gas? Yes. If enough hydrochloric acid is added to sodium carbonate, carbon dioxide is emitted as a gas.