
How to Find the Most Acidic Proton in a Molecule
HI, with a pK a of about -9, is one the strongest acids known. More importantly to the study of biological organic chemistry, this trend tells us that thiols are more acidic than alcohols.
[Solved] Which of the following compounds is most acidic? Course Hero
Which will be the most acidic hydrogen in both cases? Please explain. According to me, in the first compound 2 should be most acidic as in both 1 and 2, resonance occurs but 2's carbon is closer to the oxygen, which can stabilize the negative charge on carbon.
/common-acids-and-chemical-structures-603645_FINAL-54e6b0b3351b49dbb6cef54fd4817404.png)
10 Common Acids and Chemical Structures
Acidic nature of organic compounds can be commented on the basis of stability of the resulting conjugate base . If the conjugate base thus formed is stable then the compound is more acidic or the hydrogen is more acidic and vice versa. For option A we can see there is only one acidic hydrogen ie the hydrogen connected to the oxygen atom.

The most acidic compound among the following is
11.10: Identifying Acidic Protons. The most general principle ruling acid strength can be stated thus: strong acids have relatively stable conjugate bases. In general, the more stable the conjugate base, the stronger the acid. An important thing to remember is that stability and reactivity are inverse. The more stable a substance is, the less.
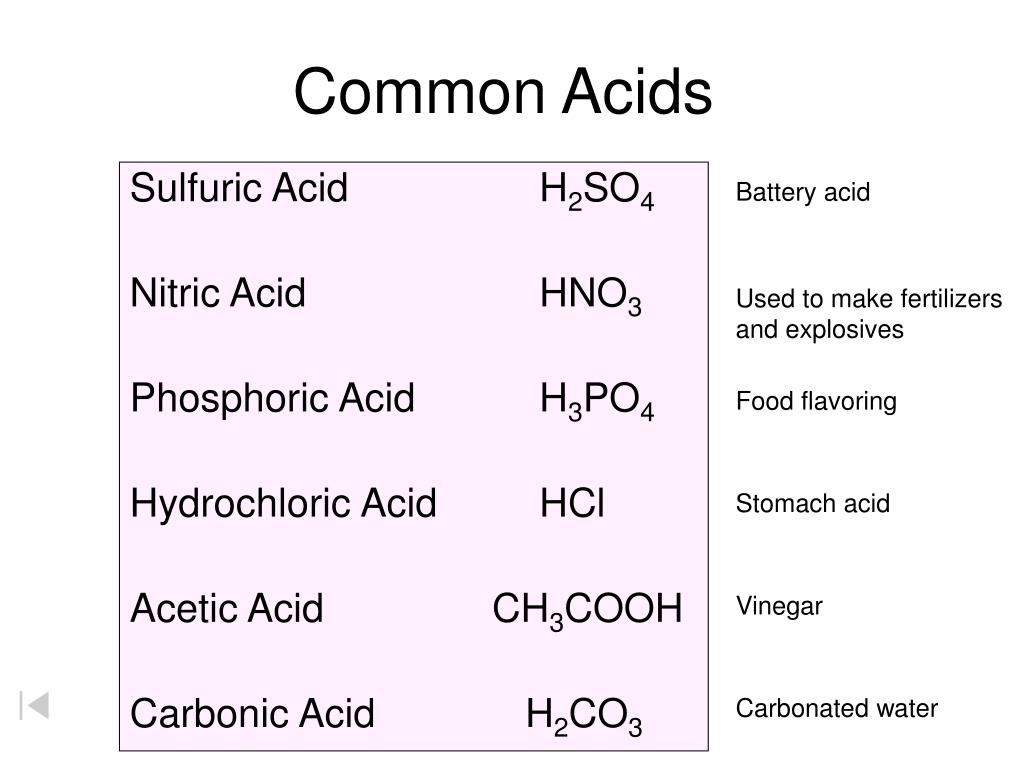
PPT Common Acids PowerPoint Presentation, free download ID4697679
Most of the acidic organic compounds are weak acids. Dissociation constant of acids (K a) value tells us about the acidity or strength of the acid. When Ka value is high, acidic strength is high. When we study about acidity of compounds, we have to look their reactions with following compounds and products and then observe reaction rates.

Which one of the following compounds is most acidic?
Tutorials Acids and Bases - How to Determine Which Acid is Stronger By Mark Coster December 1, 2021 One of the most common questions in organic chemistry is 'which of these two compounds is more acidic?' Here are the 4 most important factors to consider. How to determine the stronger acid

OneClass Identify the most acidic proton on the following compound. A) Ha B) Hb C) Hc D) Hd E
Rank the compounds below from most acidic to least acidic, and explain your reasoning. (CC-NC-SA; Timothy Soderberg via UMn Morris Digital Well) Inductive Effects. The inductive effect is an experimentally observed effect of the transmission of charge through a chain of atoms in a molecule, resulting in a permanent dipole in a bond. Inductive.
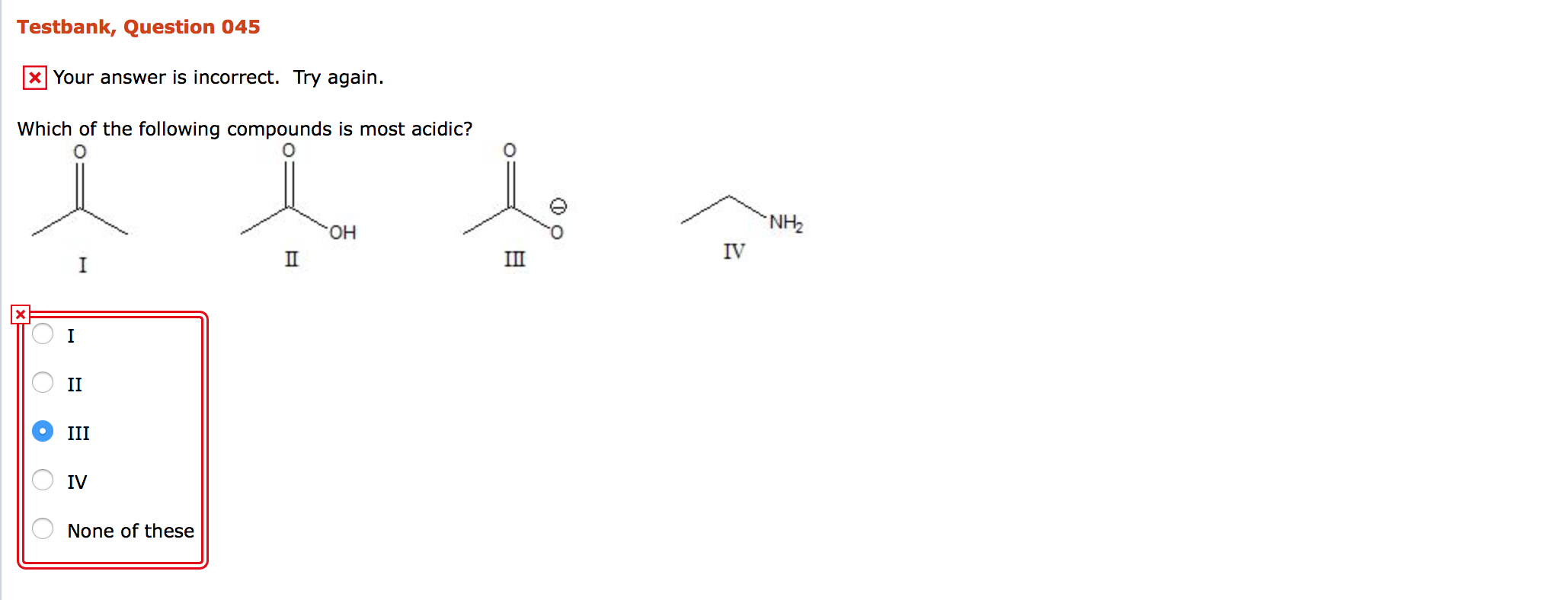
Solved Which of the following compounds is most acidic? I
The most acidic functional group usually is holding the most acidic H in the entire molecule. "Scan and rank" sounds simple, but it conceals several difficulties that are elaborated below. Scan a molecule for known acidic functional groups . Acidic protons are usually bound to O or N. Therefore, the first step is to look for all OH and NH bonds.
[Solved] For the following compounds rank the compounds from the MOST acidic... Course Hero
Carbonic acid is a chemical compound with the chemical formula H2CO3 H 2 CO 3 and is also a name sometimes given to solutions of carbon dioxide in water (carbonated water), because such solutions contain small amounts of H2CO3(aq) H 2 CO 3 ( aq). Carbonic acid, which is a weak acid, forms two kinds of salts: the carbonates and the bicarbonates.

5. The most acidic compound in the followi... Organic Chemistry
1. Factor #1 - Charge. Removal of a proton, H+ , decreases the formal charge on an atom or molecule by one unit. This is, of course, easiest to do when an atom bears a charge of +1 in the first place, and becomes progressively more difficult as the overall charge becomes negative. The acidity trends reflect this:
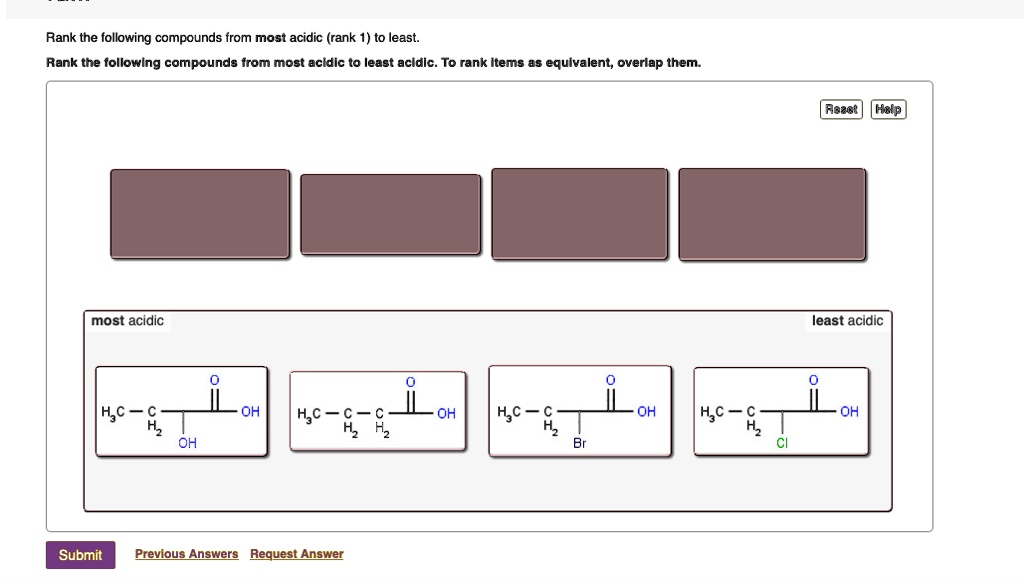
Rank the following compounds from most acidic (rank 1… SolvedLib
Positively charged molecules, or ions, are more acidic than neutral ones. Negatively charged ions tend to be basic. Examine the periodic table of elements to figure out the strength of the electronegativity. The further to the right on the periodic table the element bonded to the hydrogen is, the stronger the acid it makes.

Among the following compound , the most acidic is YouTube
Here is a list of ten common acids with chemical structures. Acids are compounds that dissociate in water to donate hydrogen ions/protons or to accept electrons . 01 of 11 Acetic Acid Acetic acid is also known as ethanoic acid. LAGUNA DESIGN / Getty Images Acetic Acid: HC 2 H 3 O 2 Also known as: ethanoic acid, CH3COOH, AcOH.

[Solved] Most acidic compound amongs the following 9to5Science
The world's strongest superacid is fluoroantimonic acid, HSbF 6. It is formed by mixing hydrogen fluoride (HF) and antimony pentafluoride (SbF 5 ). Various mixtures produce the superacid, but mixing equal ratios of the two acids produces the strongest superacid known to man. Properties of Fluoroantimonic Acid Superacid
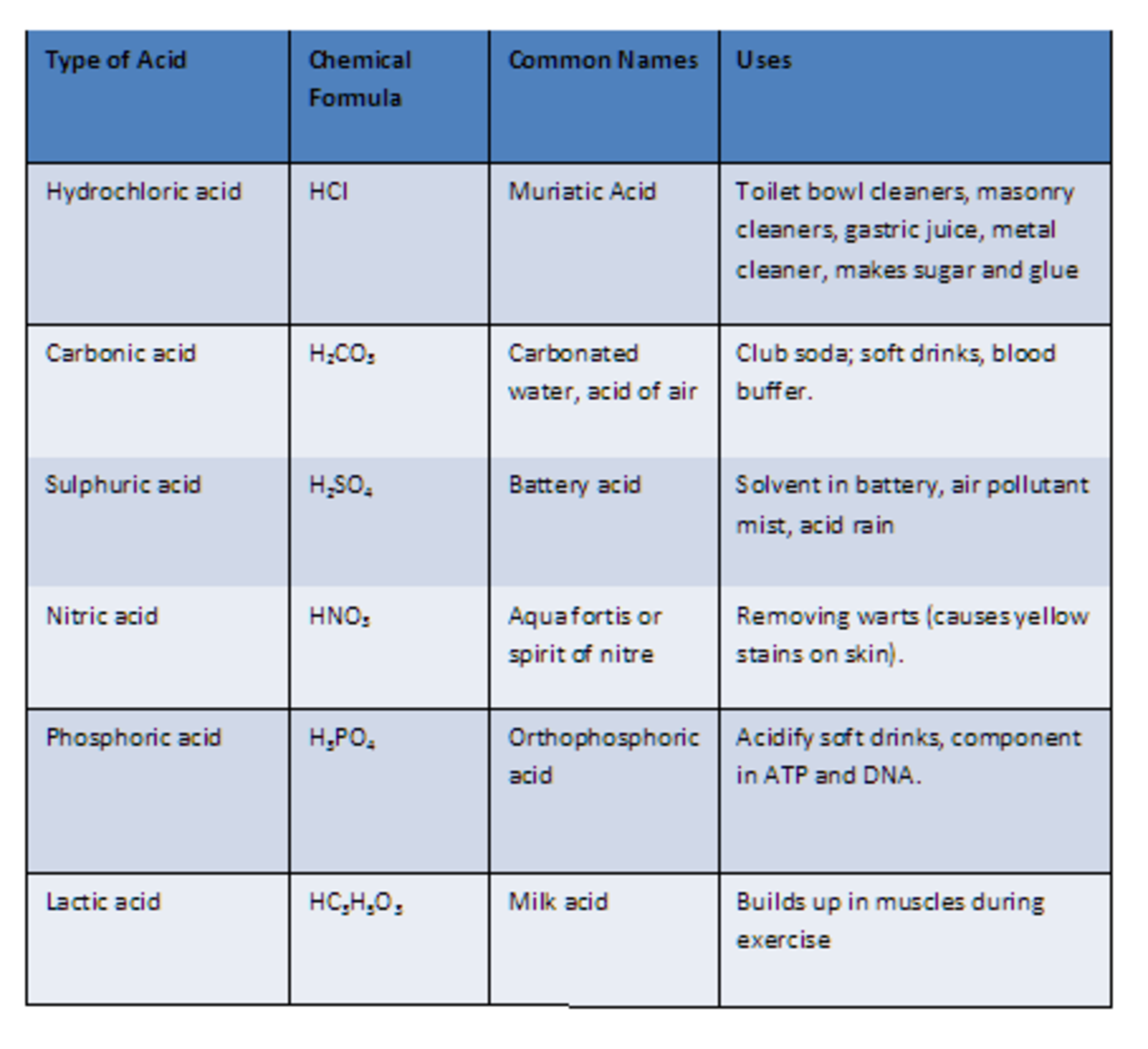
Chemical Nomenclature and Chemical Formulas Owlcation
Perhaps the most famous superacid mixture is magic acid (which does sound like it's from a movie). Invented by the grandaddy of superacid chemistry, George Olah, this comprises a 1:1 mixture of fluorosulfuric acid and antimony pentafluoride.. This super acidic system is the strongest ever measured, with a Hammett acidity function of -28.
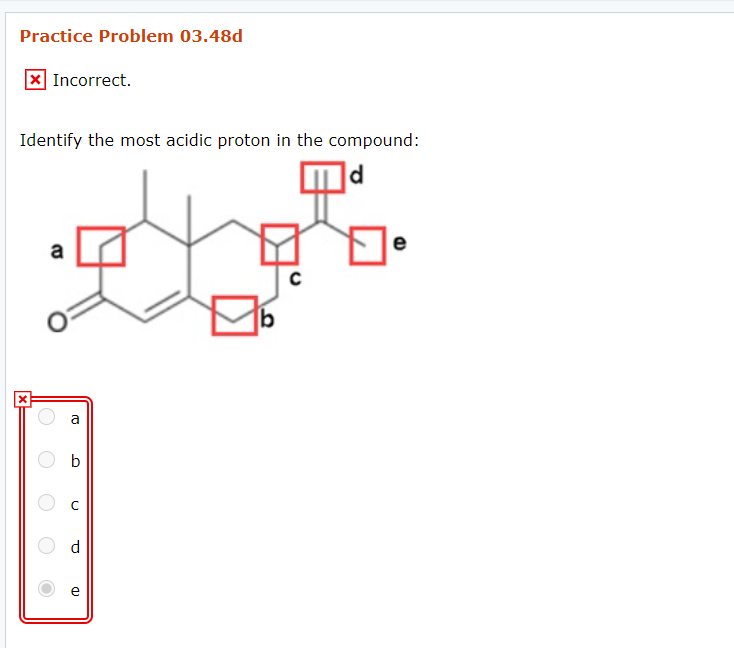
Identify the Most Acidic Proton in the Compound JohannahasBolton
For now, the concept is applied only to the influence of atomic radius on anion stability. Because fluoride is the least stable (most basic) of the halide conjugate bases, HF is the least acidic of the haloacids, only slightly stronger than acetic acid. HI, with a pK a of about -9, is one the strongest acids known.

Solved Identify the most acidic compound in this series ??
The least acidic compound (second from the right) has no phenol group at all - aldehydes are not acidic. The most acidic compound (second from the left) is a phenol with an aldehyde in the 2 ( ortho ) position, and as a consequence the negative charge on the conjugate base can be delocalized to both oxygen atoms.