
Is nitronium NO2+ ion polar or nonpolar? YouTube
N2 is a nonpolar molecule because of its linear geometrical structure and it is a diatomic molecule. As a result, both atoms have equal electronegativity and share an equal proportion of charge and the overall molecule result in a net-zero dipole moment making it a nonpolar molecule. Nitrogen, or N2, is a very abundant and necessary chemical.

Polar vs. Nonpolar Bonds — Overview & Examples Expii Ionic Bonding
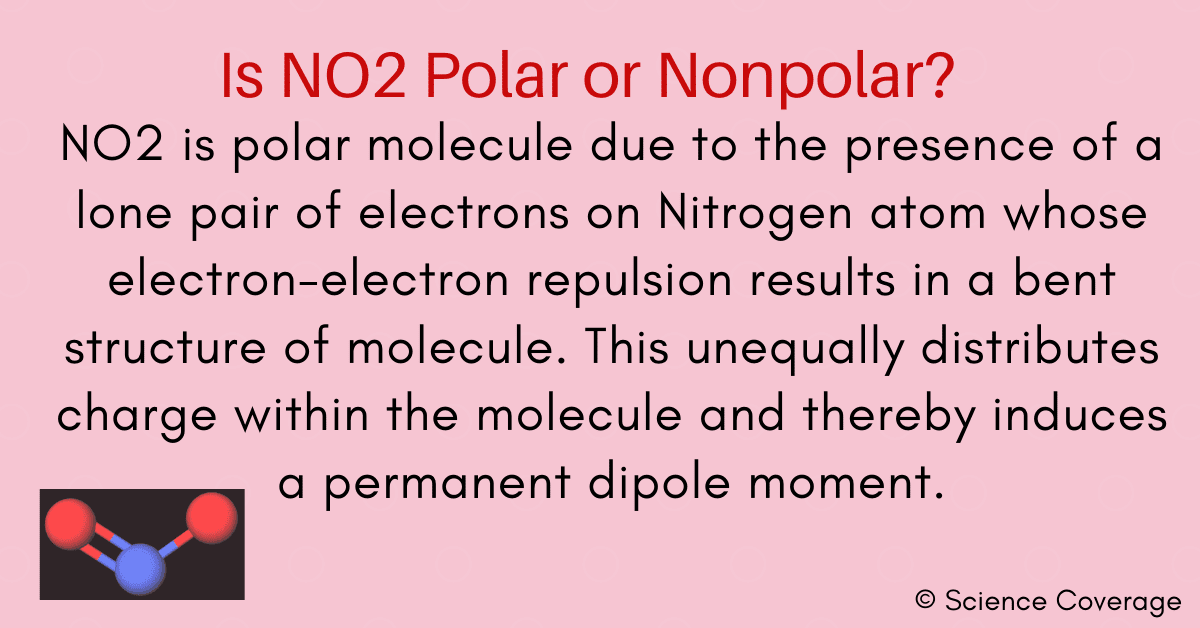
NO2 is a polar molecule and the Oxygen atom closest to negative side as the electronegativity of Oxygen (3.44) is comparatively greater than Nitrogen (3.04) so that Nitrogen has a partial positive charge and Oxygen has a partial negative charge established within the molecule.

Science Coverage Is NO2+ Polar or Nonpolar? Electron affinity
Geometry NO2 Polar or Nonpolar To determine if NO 2 is polar or nonpolar, we need to first determine its geometry. This presumes knowing the rules for drawing a correct Lewis structure and you can find more details about Lewis structures here. Nitrogen is the central atom, so we can draw the skeletal structure:
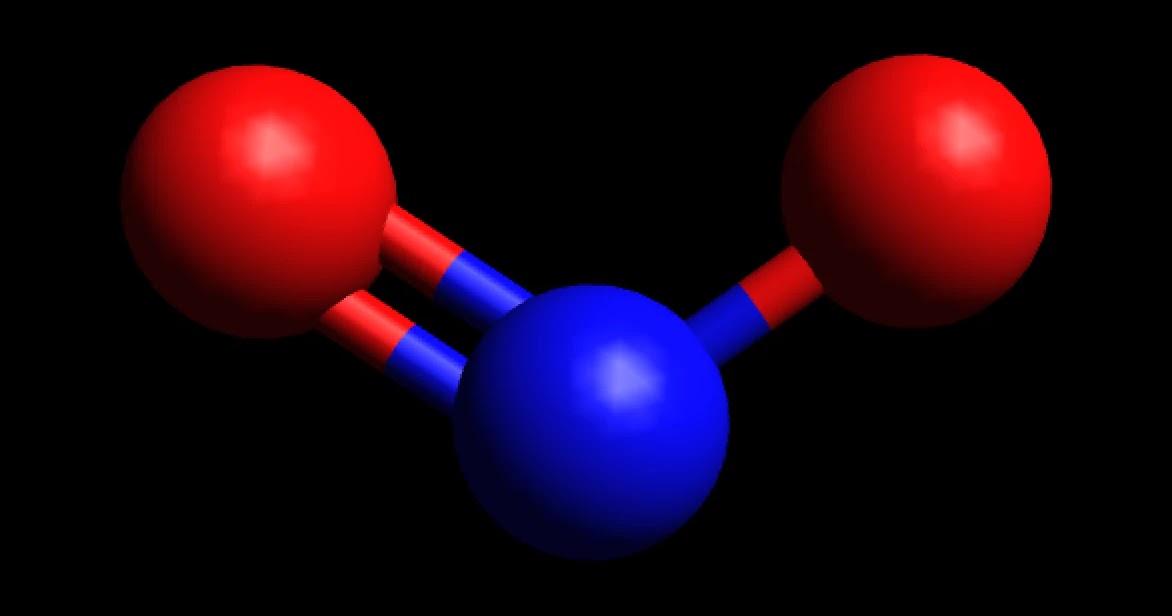
MakeTheBrainHappy Is NO2 Polar or Nonpolar?
Because the two C=O C = O bonds in COX2 C O X 2 are polarized (whereas in OX2 O X 2 the bond is not polarized) it makes it easier for the polar water molecule to solvate it and to form hydrogen bonds. Both of these factors will stabilize a COX2 C O X 2 molecule more than an OX2 O X 2 molecule in water; stabilization translates into greater.
Is NO2 Polar or Nonpolar? [Brief Explanation in simple terms]
Definition of Polar and Nonpolar Molecules. Before we delve into the polar or nonpolar nature of the NO2 Lewis structure, let's first understand what it means for a molecule to be polar or nonpolar. In chemistry, polarity refers to the distribution of electrons in a molecule. A polar molecule has an uneven distribution of electron density.

Is NO2+ (Nitronium Ion) Polar or Nonpolar? YouTube
Page Contents show What makes a molecule polar or non-polar? A molecule is polar if there is a non-uniform charge distribution present in it. If the charge distribution gets equally balanced in different parts, then that molecule or molecular ion is considered non-polar.

Is N2O Polar or Nonpolar? YouTube
On the other hand, non-polar molecules are those where atoms shared an equal number of electrons or where polarity will be canceled out in a molecule. In the NO2 molecule, the oxygen atom is more electronegative than the nitrogen atom, hence the oxygen atom pulls electrons towards itself. Therefore, the N-O bond is polar in nature.

Poláris és nem poláris molekulák Free Press
NO2+ (Nitronium ion) is nonpolar in nature because it has a linear geometrical structure due to which polarity of opposite NO bonds gets canceled by each other resulting in the nonpolar NO2+ ion. Nitronium ion is a stable ion in normal conditions. But it is also widely used as an electrophile in the nitration of various compounds.

Is NO2 Polar or Nonpolar? (Nitrogen dioxide) YouTube
Learn to determine if N2 is polar or nonpolar based on the Lewis Structure and the molecular geometry (shape).We start with the Lewis Structure and then use.

Properties of NO2+
If you look at the Lewis structure for N2O it appears to be a symmetrical molecule. However, to determine if N2O is polar we consider the molecular geometry.

Is the Nitronium Ion (NO2+) Polar or Non Polar? Lewis Structure YouTube
Learn to determine if NO2 + is polar or nonpolar based on the polarity between bonds and the molecular geometry (shape).Ions, like NO2+ are sometimes confusi.

Best overview Is NO2+ Polar or Nonpolar 1
Published by on NO2 polar or nonpolar - No2 is an organic chemical compound. the chemical name of no2 is nitrogen dioxide. and no2 is polar molecules. Hello, reders welcome to another fresh article, today, we will discuss about no2 polar or nonpolar and charge, molar mass, structure . We provide valuable information regarding this topic.
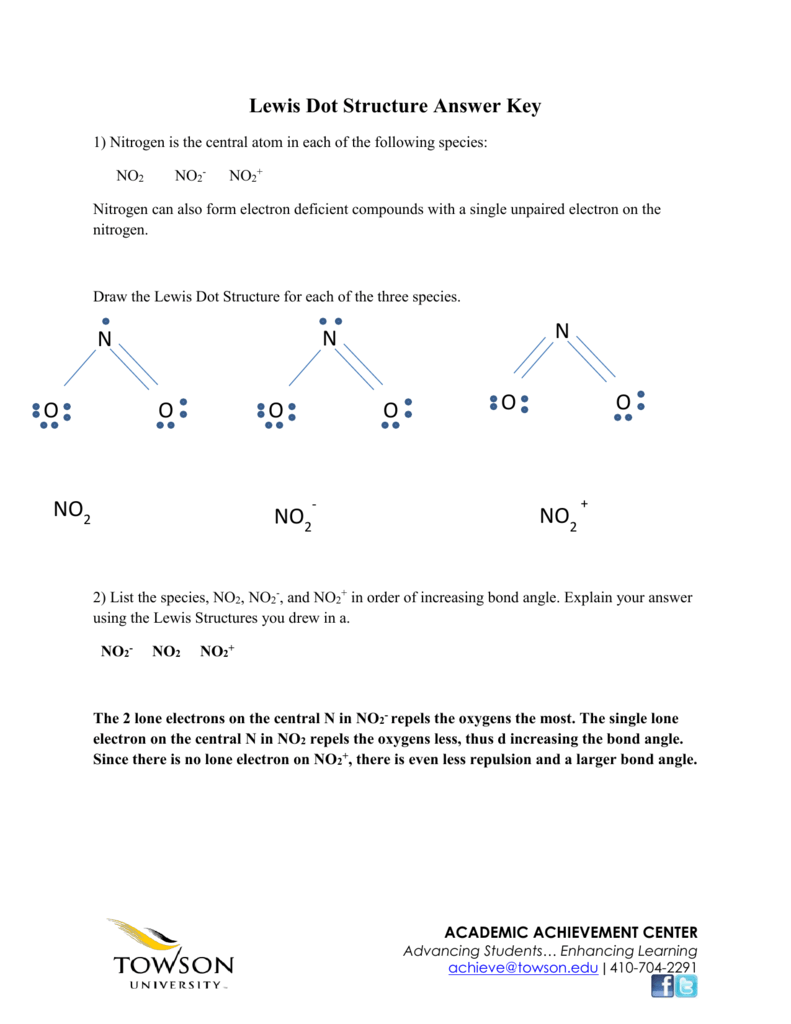
Answer Key
Home Chemistry Is NO2+ Polar or Nonpolar? by Richard - October 07, 2020 0 NO2+ is a nonpolar molecule despite two N-O bonds are polar. NO2+ has a linear geometry due to no lone pair of electrons on central N atom which causes cancellation of both positive and negative charges produced on the molecule, as a result, the net dipole becomes zero.

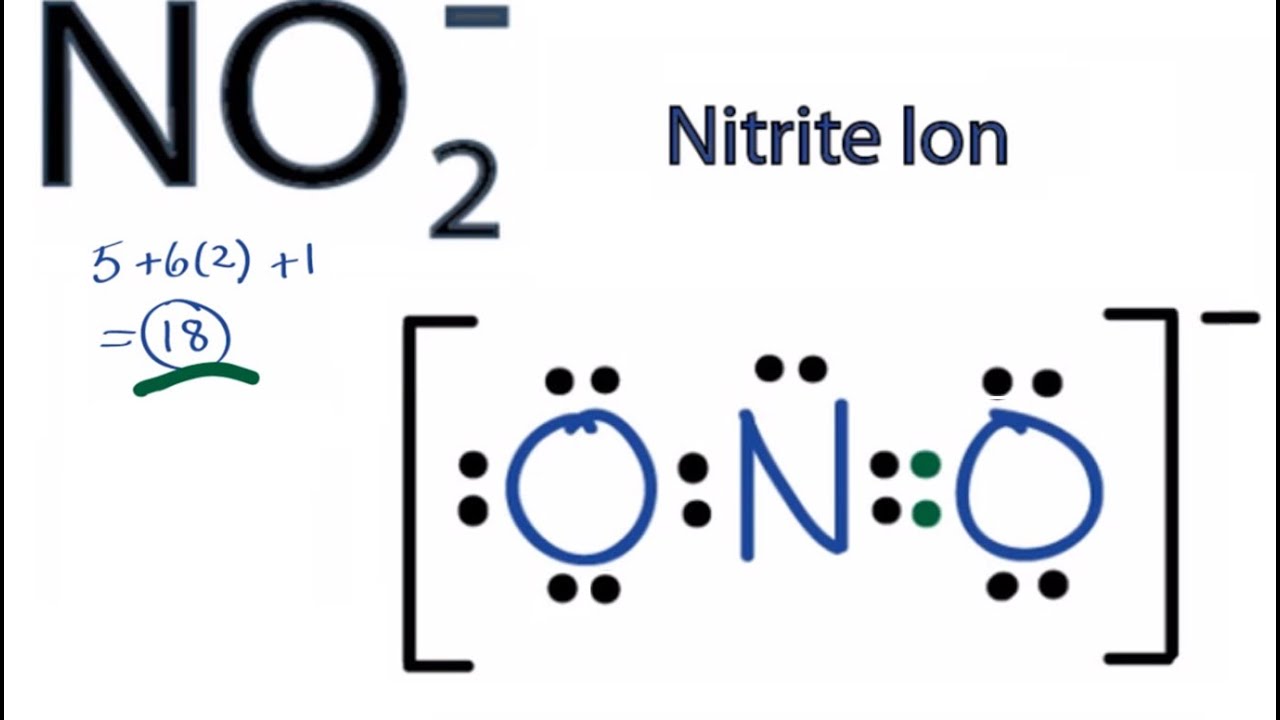
Draw Lewis structure for NO2^ + (nitronium ion).
Want to know the reason? Let's dive into it! NO2 is a POLAR molecule because it has one unpaired electron on the Nitrogen atom (N) which causes the entire molecule to bend. This bending of NO2 molecule results in asymmetric geometry, which makes the molecule polar.
ハンドメイ No.2の通販 by DREAM STAR's shop|ラクマ カテゴリ
When the difference is very small or zero, the bond is covalent and nonpolar. When it is large, the bond is polar covalent or ionic. The absolute values of the electronegativity differences between the atoms in the bonds H-H, H-Cl, and Na-Cl are 0 (nonpolar), 0.9 (polar covalent), and 2.1 (ionic), respectively.

NO2F Lewis Structure, Molecular Geometry, Hybridization, and Polarity
The molecule is symmetric. The two oxygen atoms pull on the electrons by exactly the same amount. Propane is nonpolar, because it is symmetric, with H atoms bonded to every side around the central atoms and no unshared pairs of electrons. Exercise 4.12. 1. Label each of the following as polar or nonpolar.