
Step2 Lewis Structure of PH3 for constructing around the central Phosphorous atom
Step #1: Calculate the total number of valence electrons Here, the given molecule is PH3. In order to draw the lewis structure of PH3, first of all you have to find the total number of valence electrons present in the PH3 molecule. (Valence electrons are the number of electrons present in the outermost shell of an atom).

How to draw PH3 Lewis Structure? 4
---- Steps to Write Lewis Structure for compounds like PH3 ---- 1. Find the total valence electrons for the PH3 molecule. 2. Put the least electronegative atom in the center. Note: Hydrogen.
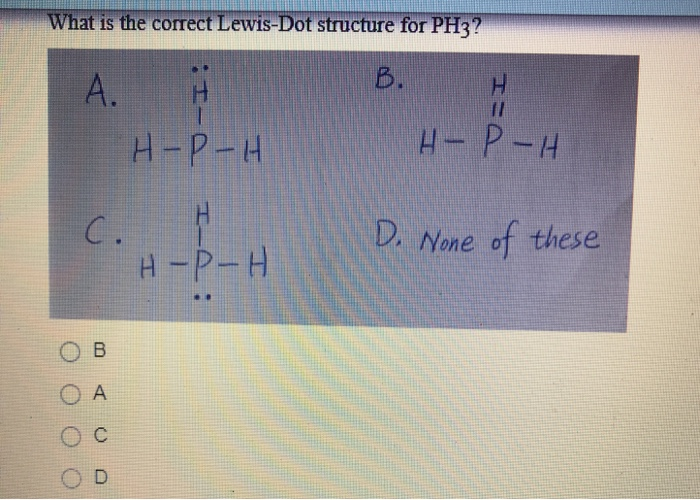
Solved What is the correct LewisDot structure for PH3? А. Н
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".
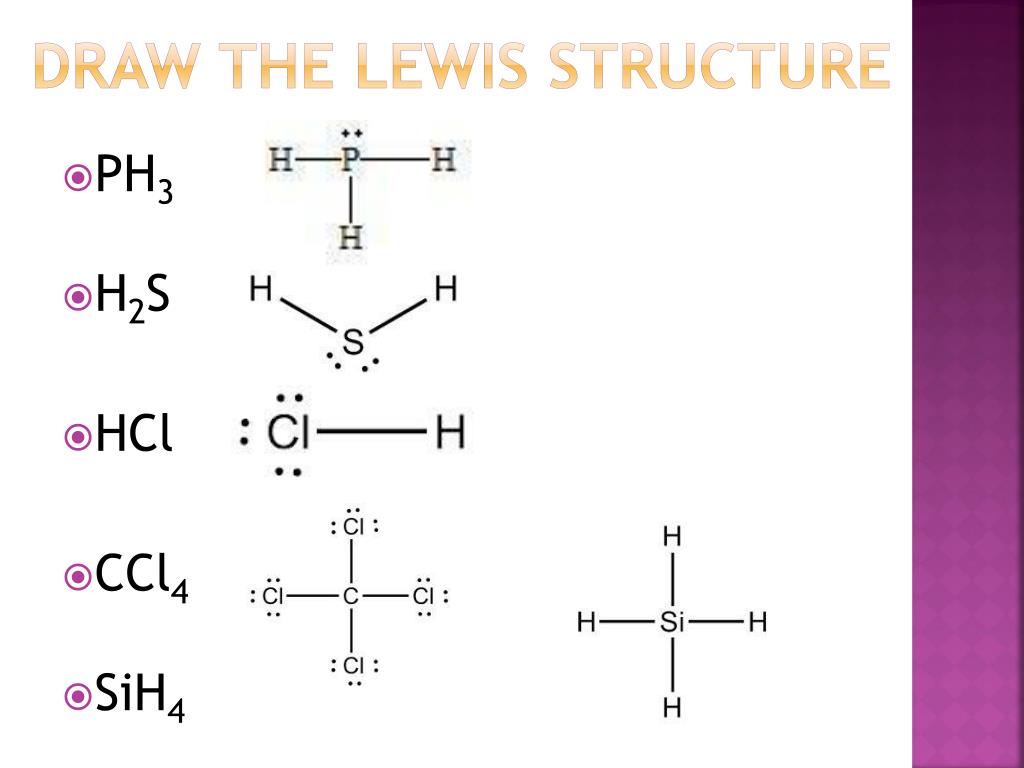
PPT Lewis Structures PowerPoint Presentation, free download ID6114495
A Lewis electron dot diagram (or electron dot diagram, or a Lewis diagram, or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the.

Ph3 Estructura De Lewis Compuesto
We can illustrate the formation of a water molecule from two hydrogen atoms and an oxygen atom using Lewis dot symbols: The structure on the right is the Lewis electron structure, or Lewis structure, for H 2 O. With two bonding pairs and two lone pairs, the oxygen atom has now completed its octet. Moreover, by sharing a bonding pair with oxygen.
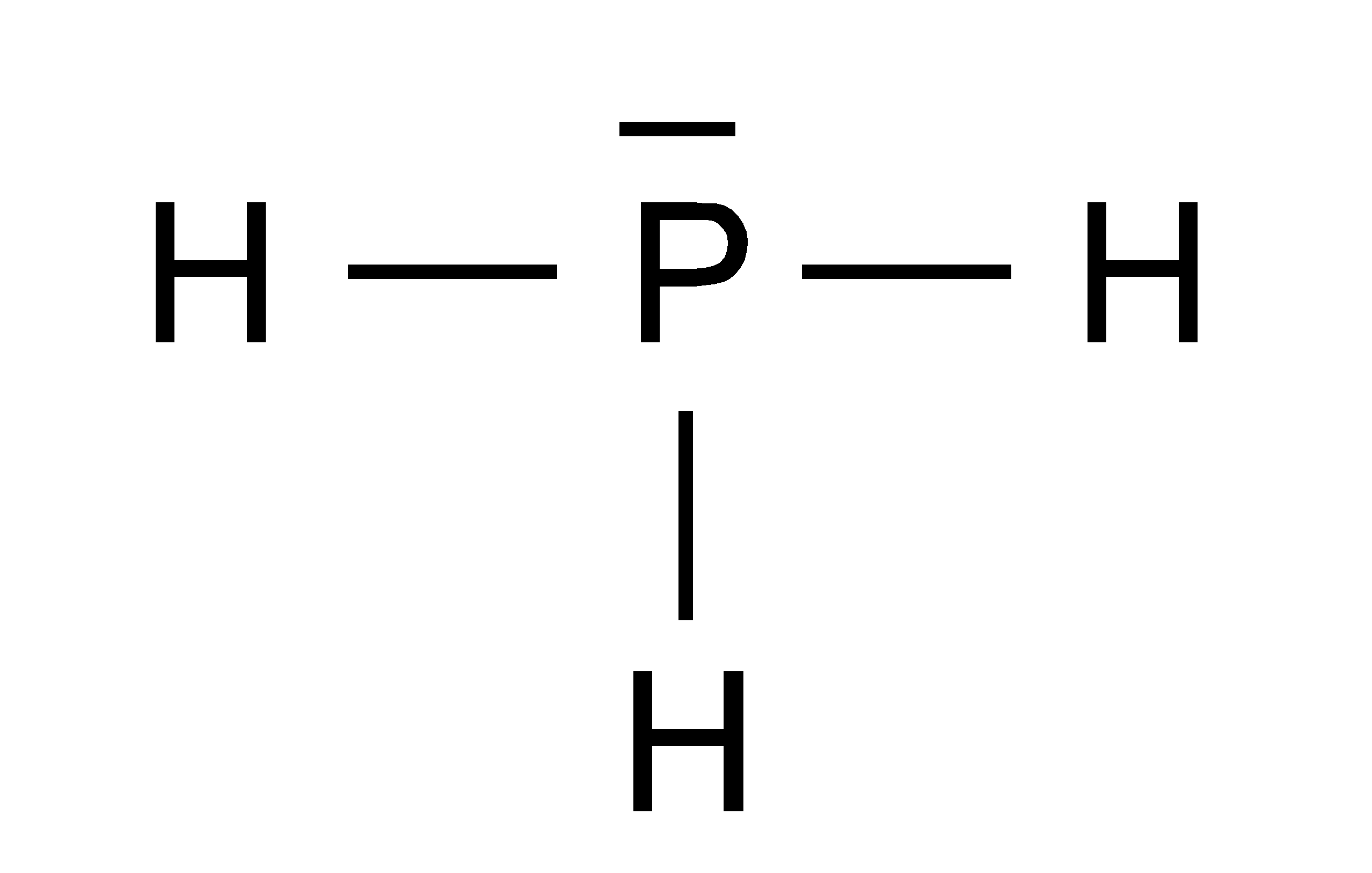
Ph3 Lewis Structure Shape
To use the Lewis Structure Calculator follow these steps: Enter the formula of the molecule in the field provided for it. For example, if we want to obtain the Lewis structure of the Sulfate ion, SO 4 - 2, we must first enter the charge by typing (-2) or by entering -2 in the charge field and pressing the «Add» button. Then we write the rest of the formula being as follows: (-2)SO4.

Ph3 Estructura De Lewis Estudiar
Lewis Symbols. We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 7.9 shows the Lewis symbols for the elements of the third period of the periodic table.

PH3 Lewis StructureLewis Structure of PH3 (Phosphorus Trihydride)Draw Lewis Structure for PH3
Step-3: Lewis dot Structure for PH3 generated from step-1 and step-2. Connect the exterior and core central atom of the PH3 molecule with three single bonds (P-H). In this stage, use three hydrogen atoms on the outside of the PH3 molecule to the central . Phosphorous atom in the middle.

Lewis dot structure of PH3 (phosphine)... YouTube
Phosphine PH3 Lewis Dot Structure shadowboy220 1.9K subscribers 49K views 11 years ago Chemistry Lewis Dot Structures A video explanation of how to draw the Lewis Dot Structure for.

Ph3 Lewis Structure Shape
The compound Phosphorous Trihydride (PH3), also known as phosphine consists of phosphorus and hydrogen atoms. It is an inflammable and toxic gas without any color. Phosphine does not have any odor when it is pure, but most samples of the gas have the unpleasant odor of rotten garlic or decaying fish.

PH3 Lewis structure YouTube
Lewis Structure is the pictorial representation of the arrangement of atoms and valence electrons in the molecule. To know the Lewis Structure, we first know the central atom and the arrangement of other atoms. Here for PH3, the phosphorus atom will take the central position as Hydrogen atoms cannot take a central position in the Lewis Structure.
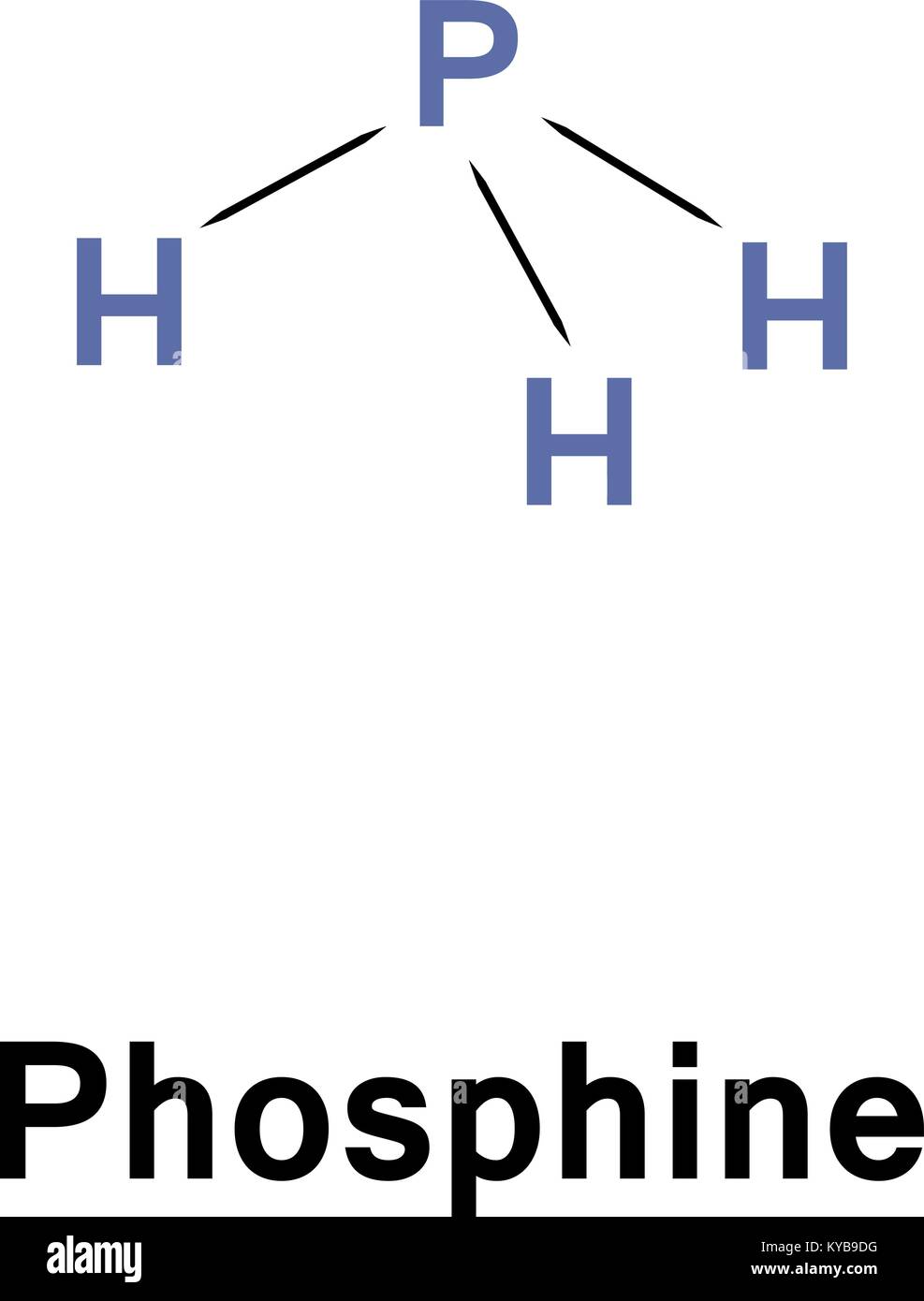
Ph3 Lewis Structure Shape
Now in the PH3 molecule, you have to put the electron pairs between the phosphorus atom (P) and hydrogen atoms (H). This indicates that the phosphorus (P) and hydrogen (H) are chemically bonded with each other in a PH3 molecule. Step 4: Make the outer atoms stable. Place the remaining valence electrons pair on the central atom.

Draw The Lewis Structure Of Ph3
1×5 + 3×1 = 8. The trial structure has exactly the same number of electrons as we have available. Therefore, the trial structure is the correct Lewis structure. Answer link Here are the steps that I follow when drawing a Lewis structure. Decide which atom is the central atom in the structure.

Ph3 Estructura De Lewis Estudiar
Steps for drawing the Lewis dot structure for PH3 1. Count total valence electron in PH3 First of all, determine the valence electron that is available for drawing the lewis structure of PH 3 because the lewis diagram is all about the representation of valence electrons around atoms.

PH3 Lewis Structure How to Draw the Lewis Structure for PH3 YouTube
Identify each violation to the octet rule by drawing a Lewis electron dot diagram. ClO; SF 6; Solution. a. With one Cl atom and one O atom, this molecule has 6 + 7 = 13 valence electrons, so it is an odd-electron molecule. A Lewis electron dot diagram for this molecule is as follows:
PH3 Lewis Structure, Molecular Geometry, Hybridization, Bond Angle and Shape
In this tutorial we will learn how to draw the lewis structure of PH 3 and determining the shape and molecular geometry of the molecule. PH 3 lewis structure According to the lewis structure shown above, you will understand phosphine's structure is a simple. Therefore, we can draw the lewis structure of phosphine easily.