
What Is Pcl3 Lewis Structure?
In a polar covalent bond, sometimes simply called a polar bond, the distribution of shared electrons within the molecule is no longer symmetrical (see figure below). Figure 5.3.4 5.3. 4: In the polar covalent bond of HF HF, the electron density is unevenly distributed. There is a higher density (red) near the fluorine atom, and a lower density.
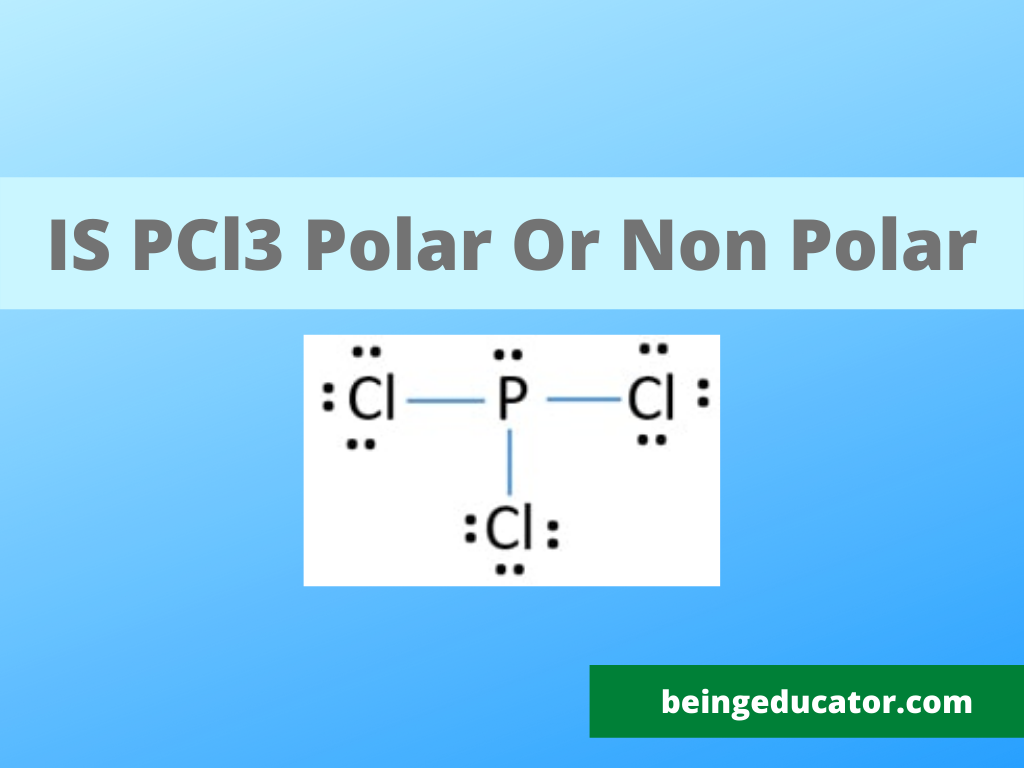
Pcl3 Structure
PCl 3 is a polar molecule because the P-Cl bond is polar, and the three bonds are not equivalent to the lone pair which causes an asymmetrical distribution of bonding electrons in the molecule. This results in a permanent dipole directed towards the P-Cl bonds as drawn: Check this 99-question multiple-choice quiz on Geometry and Hybridization: Free

PCl3 Lewis Structure, Hybridization, Molecular Geometry, and MO Diagram
Wayne Breslyn Join this channel and unlock members-only perks Learn to determine if PCl3 (Phosphorous trichloride) is polar or non-polar based on the Lewis Structure and the molecular.

Polar and Nonpolar Molecules
Despite the polar bonds, PCl3 is a nonpolar molecule due to its symmetrical arrangement. PCl3 Lewis Structure Bond Angle. The bond angle in PCl3, or phosphorus trichloride, is a crucial aspect of its molecular geometry. Understanding the bond angle helps us comprehend the overall shape and properties of the molecule.
Is PCl3 (Phosphorous trichloride) Polar or NonPolar YouTube
Hey Guys!In this video, we are going to determine the polarity of Phosphorus Trichloride having a chemical formula of PCL3.To know the polarity of this molec.

Pcl3 Polar Or Nonpolar Asking List
Is PCl3 polar or nonpolar? Don't worry, the answer is simple! PCl3 is a polar molecule because of its geometry and difference in electronegativity between the 2 atoms. Again another question like, whether PCl3 is ionic or covalent, can pop up in your mind.

Is PCL3 Polar or Nonpolar? (Phosphorus Trichloride) YouTube
Formula: The chemical formula of phosphorous trichloride is (PCL3). Common Name: The common name of PCL3 is Trichlorophosphane, Phosphorous chloride, and Phosphorus (III) chloride. Table of Contents Is PCL3 Polar Or Nonpolar Summary PCL3 Molecular Geometry Summary PCL3 Lewis Structure Hybridization Of PCL3 Summary Bond Angle Of PCL3 Summary

How to draw PCl3 Lewis Structure? Science Education and Tutorials
Molecular Polarity. To determine if a molecule is polar or nonpolar, it is frequently useful to look at Lewis structures. Nonpolar compounds will be symmetric, meaning all of the sides around the central atom are identical - bonded to the same element with no unshared pairs of electrons.Polar molecules are asymmetric, either containing lone pairs of electrons on a central atom or having atoms.

Is PCl3 (Phosphorus trichloride) Ionic or Covalent/Molecular? YouTube
The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. Calculate the electronegativity difference (ΔEN) and average ( EN) of the two electronegativities, and use the table below to determine the bond type and polarity. Calculate the molecular polarity (polar, non-polar) of a chemical bond based.
How To Know If A Molecule Is Polar Or Nonpolar Khan Academy
Phosphorus trichloride is made up of one Phosphorus atom and three Chlorine atoms, having a chemical formula of PCl3. It is a volatile liquid that reacts with water and releases HCl gas. It is a toxic compound but is used in several industries. Phosphorus Trichloride is widely used in manufacturing Phosphites and other organophosphorus compounds.

Is PCl3 Polar or NonPolar? (Phosphorous trichloride) Yes Dirt
PCl3 is a POLAR molecule because the Chlorine (Cl) present in the molecule is more electronegative, which causes the partial positive (ẟ+) and partial negative (ẟ-) charge to appear on the molecule. These ẟ+ and ẟ- charges are responsible to make the entire PCl3 molecule polar.

PCl3 Lewis Structure and Molecular Geometry YouTube
Conclusion FAQ on "Is PCl3 polar or nonpolar?" Is PCl3 a polar compound? What type of bond is PCl3? Is PCl3 a dipole?

Polar and Nonpolar Covalent Bonds Characteristics & Differences
In order to know whether PCl3 is a polar covalent molecule or nonpolar covalent molecule, we have to check the electronegativity difference of the combining atoms. If the electronegativity difference ( ΔEN ) is less than 0.4 , then the bond is nonpolar covalent bond.
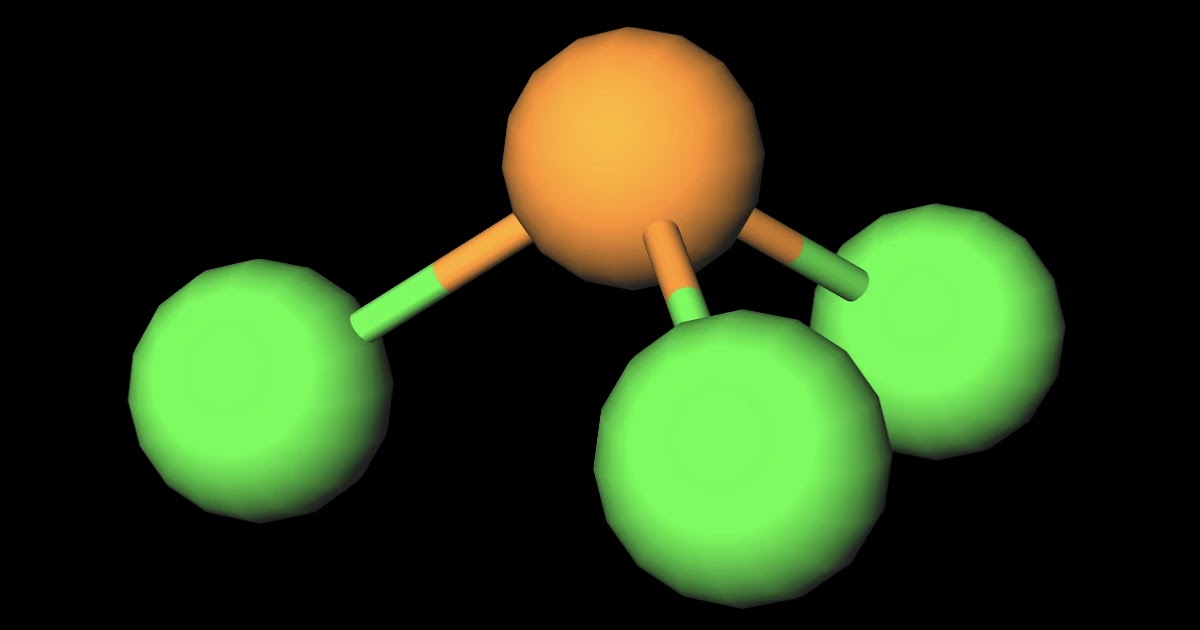
MakeTheBrainHappy Is PCl3 Polar or Nonpolar?
POCl3 is a POLAR molecule because the P=O bond and P-Cl bonds present in the molecule are polar and it has asymmetric geometry which causes the partial positive (ẟ+) and partial negative (ẟ-) charge to appear on the molecule. These ẟ+ and ẟ- charges are responsible to make the entire POCl3 molecule polar.

Is \ce{PCl3} polar or nonpolar? Quizlet
Phosphorous trichloride molecules are polar because each atom contributes unevenly to the molecule's electron density, making it unequally charged along its three axes.

PCl3 Lewis Structure, Hybridization, Molecular Geometry, and MO Diagram
The PCl3 is a polar molecule, and the polarity is due to its tetrahedral geometry with a lone pair of electrons on the phosphorus atom. The electronegativity difference between chlorine and phosphorus creates two opposing dipoles with a more negative charge on chlorine and a positive charge on phosphorus results in polar bond formation.