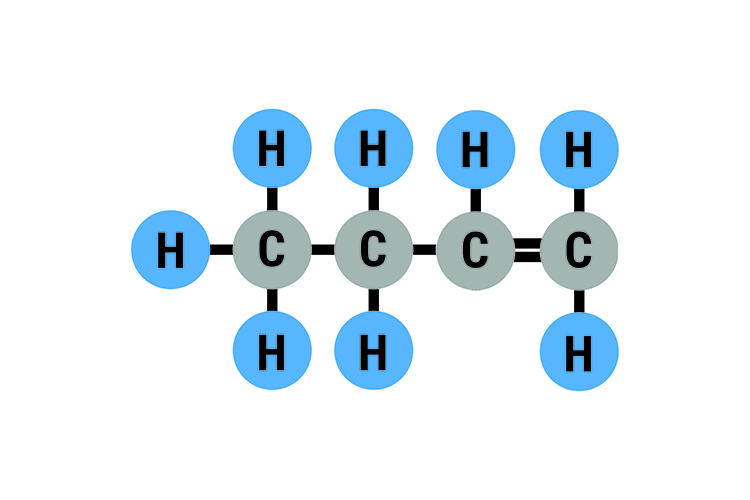
The molecular structure of Butene and formula structure
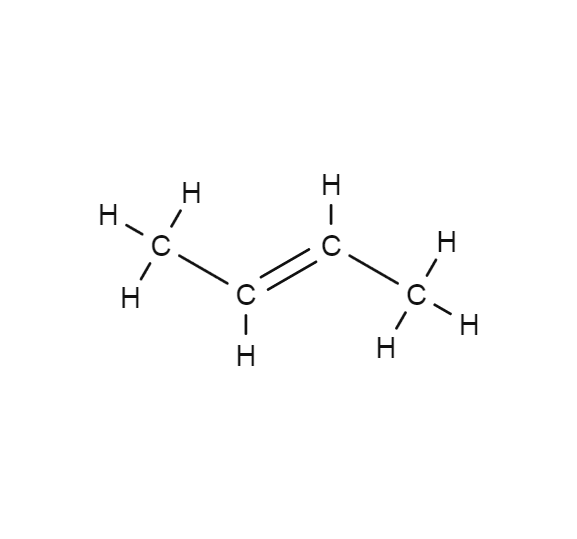
2-Butene is a hydrocarbon consisting of Carbon (C) and Hydrogen (H) a. In this video we'll write the structural formula for 2-Butene (also called But-2-ene). 2-Butene is a hydrocarbon.
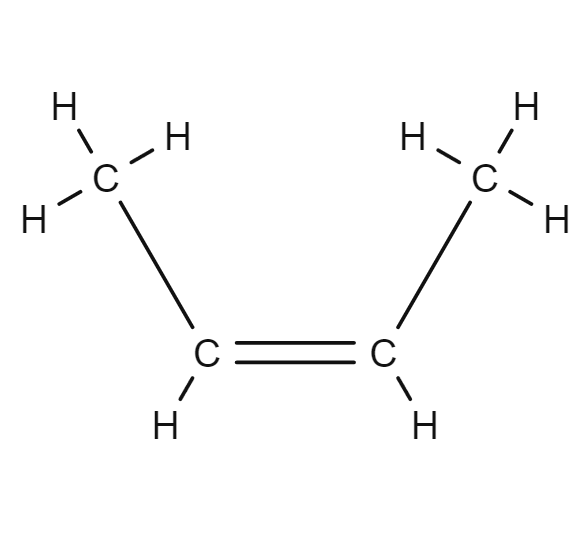
2Butene Structural Formula Butene compound cis hydrogenation minimum trans heat which October
Butene, also known as butylene, is an alkene with the formula C 4 H 8.The word butene may refer to any of the individual compounds. They are colourless gases that are present in crude oil as a minor constituent in quantities that are too small for viable extraction. Butene is therefore obtained by catalytic cracking of long-chain hydrocarbons left during refining of crude oil.

What is the Difference Between 1 Butene and 2 Butene Compare the Difference Between Similar Terms
Reactivity Profile. The unsaturated aliphatic hydrocarbons, such as 2-BUTENE, are generally much more reactive than the alkanes. Strong oxidizers may react vigorously with them. Reducing agents can react exothermically to release gaseous hydrogen. In the presence of various catalysts (such as acids) or initiators, compounds in this class can.
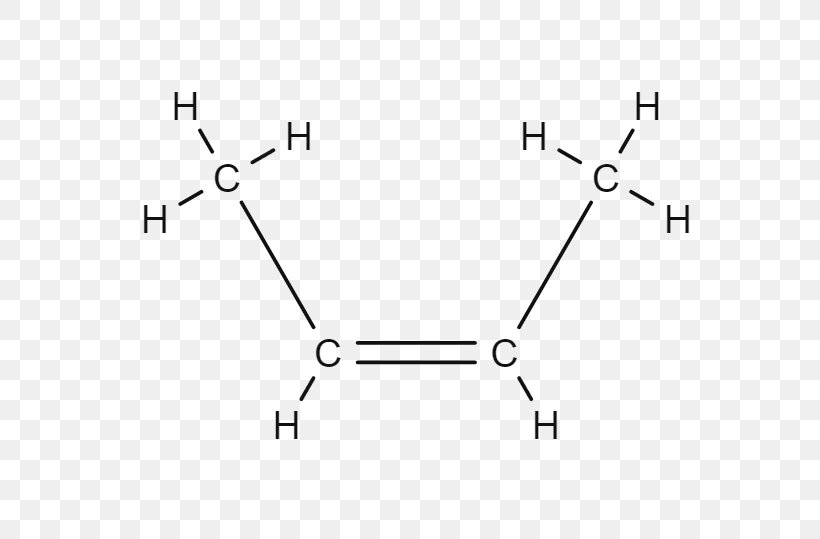
2Butene 1Butene Cistrans Isomerism Alkene, PNG, 577x539px, Butene, Air Liquide, Alkane
But-2-ene ( / ˈbjuːt.tu.iːn /) is an acyclic alkene with four carbon atoms. It is the simplest alkene exhibiting cis / trans -isomerism (also known as ( E / Z )-isomerism); that is, it exists as two geometric isomers cis -but-2-ene ( ( Z )-but-2-ene) and trans- but-2-ene ( ( E )-but-2-ene). It is a petrochemical, produced by the catalytic.
50 best ideas for coloring R Isomer
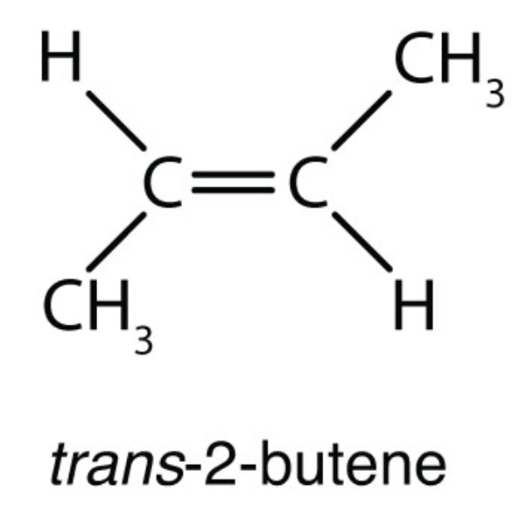
Therefore, this is (Z)-2-butene. Now look at the right hand structure (the trans isomer). In this case, the priority group is "down" on the left end of the double bond and "up" on the right end of the double bond. Since the two priority groups are on opposite sides of the double bond, they are entgegen = opposite. Therefore, this is (E)-2-butene.
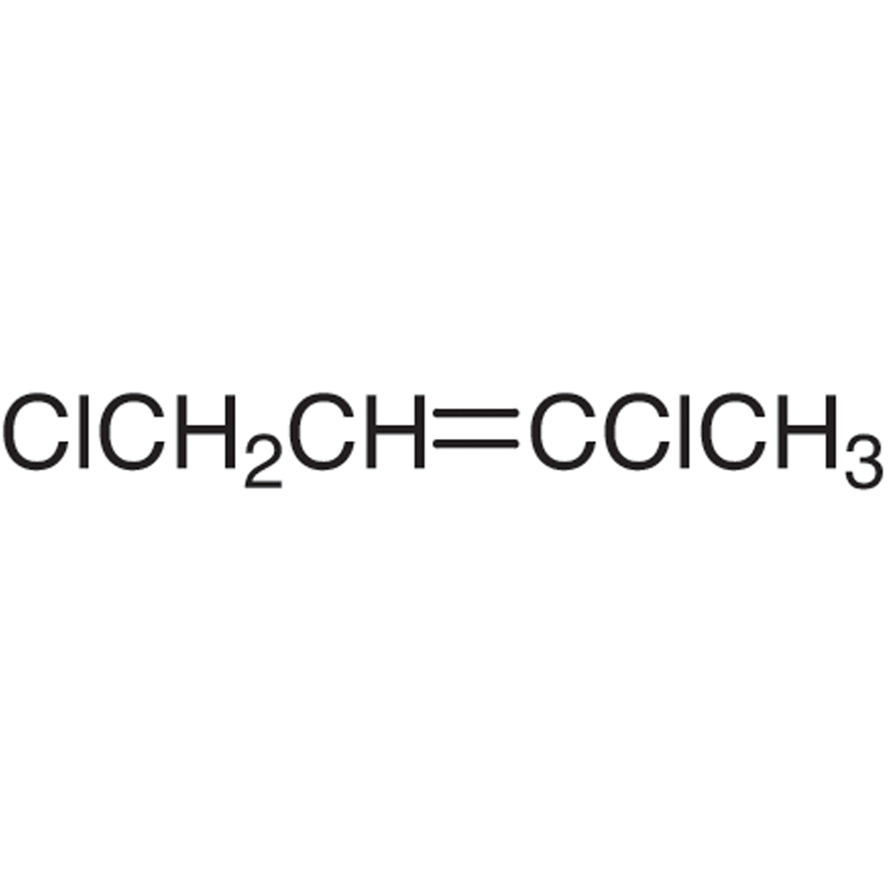
1,3Dichloro2butene (cis and trans mixture) 3BD0351
2-Butene, (Z)-2-Butene, (E)-Other names: β-Butene; β-Butylene; Pseudobutylene; CH3CH=CHCH3; But-2-ene Permanent link for this species. Use this link for bookmarking this species for future reference. Information on this page: Mass spectrum (electron ionization) References; Notes; Other data available: Gas phase ion energetics data; IR Spectrum
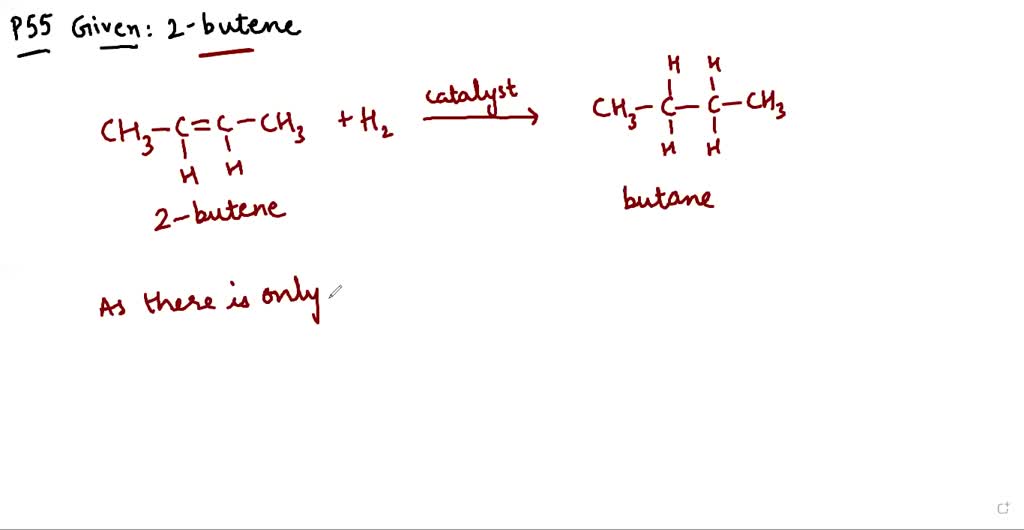
SOLVEDWrite an equation for the hydrogenation of 2butene. Does the cis or trans geometry of
Quantity Value Units Method Reference Comment; Δ r H°-118.5 ± 0.42: kJ/mol: Chyd: Kistiakowsky, Ruhoff, et al., 1935: gas phase; Reanalyzed by Cox and Pilcher, 1970, Original value = -119.54 ± 0.079 kJ/mol; At 355 °K
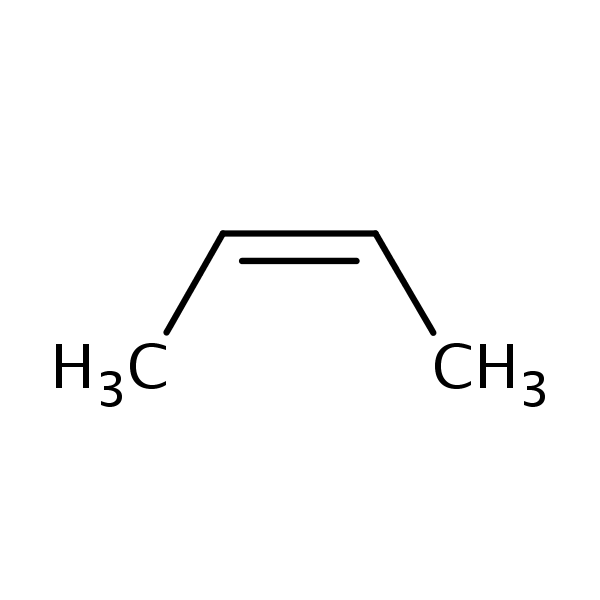
2Butene, (2Z) SIELC Technologies
Therefore, this is (Z)-2-butene. Now look at the right hand structure (the trans isomer). In this case, the priority group is "down" on the left end of the double bond and "up" on the right end of the double bond. Since the two priority groups are on opposite sides of the double bond, they are entgegen = opposite. Therefore, this is (E)-2-butene.

2 Methyl 2 Butanol Synthesis
2-Butene's production and use in the production of gasolines, butadiene and other chemicals(1) may result in its release to the environment through various waste streams(SRC). 2-Butene is an isomeric mixture of trans-and cis-2-butene recovered from refining gases or produced by petroleum cracking(2). 2-Butene occurs in coal gas and has been.
[Solved] Some 2methyl 2butene may be produced during the reaction. Explain... Course Hero
The 2-butene isomer in which the two methyl groups are on the same side is called a cis-isomer; the one in which the two methyl groups are on opposite sides is called a trans-isomer (Figure 20.10). The different geometries produce different physical properties, such as boiling point, that may make separation of the isomers possible:.
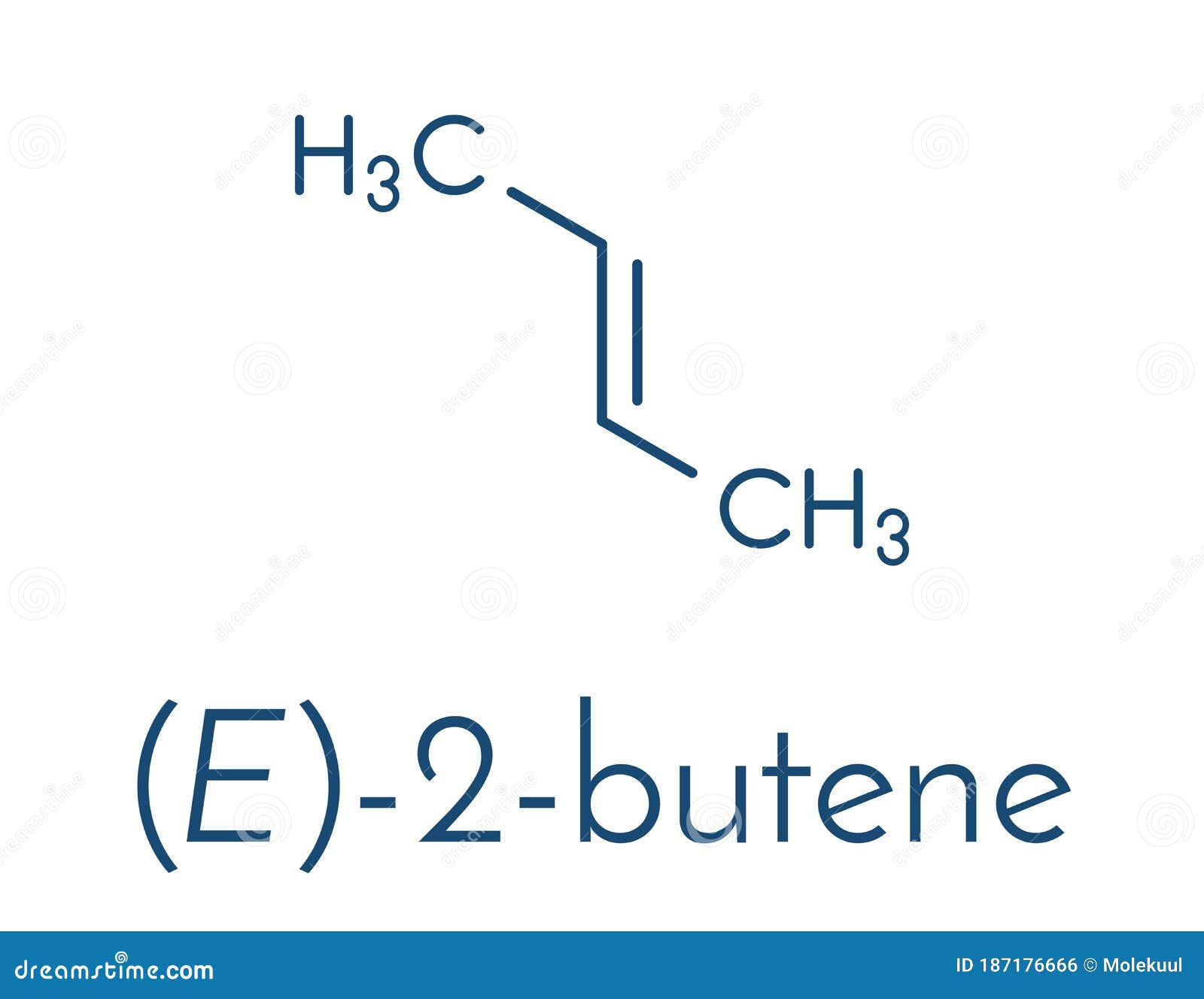
2butene Trans, Eform Molecule. Common Petrochemical. Skeletal Formula. Stock Vector
Geometric Isomers of 2-Butene. The cis isomer has the two single hydrogen atoms on the same side of the molecule, while the trans isomer has them on opposite sides of the molecule. In both molecules, the bonding order of the atoms is the same. In order for geometric isomers to exist, there must be a rigid structure in the molecule to prevent.
Trans 2 Butene Structure
25.5: Isomers. As we delve into the complexities of organic chemistry, we will see how molecular shape affects reactions. One common reaction for alkenes is the addition of hydrogen across the double bond to form the corresponding alkane. Because of the geometry of the reaction, the different 2-butene shapes have different heats of reaction.
2 Butene Alchetron, The Free Social Encyclopedia
In isomerism: Cis and trans forms.alkenes, two versions of 2-butene exist. They are traditionally called cis-2-butene and trans-2-butene or, in slightly more modern terms, (Z)- and (E)-2-butene.The Z and E stand for the German words for "together" (zusammen) and "apart" (entgegen).In principle, cis- and trans-2-butene are conformational isomers; in theory, they could…

draw a correct structure for 2 butene howtoposeforpicturesblackgirl
Butan-2-ol, or sec-butanol, is an organic compound with formula C H 3 CH(OH)CH 2 CH 3.Its structural isomers are 1-butanol, isobutanol, and tert-butanol. 2-Butanol is chiral and thus can be obtained as either of two stereoisomers designated as (R)-(−)-butan-2-ol and (S)-(+)-butan-2-ol.It is normally encountered as a 1:1 mixture of the two stereoisomers — a racemic mixture.

Name Two Isomers Of Butane kulturaupice
Iso-butene (iso-C4H8) is an important raw material in chemical industry, whereas its efficient separation remains challenging due to similar molecular properties of C4 olefins. The ideal adsorbent.
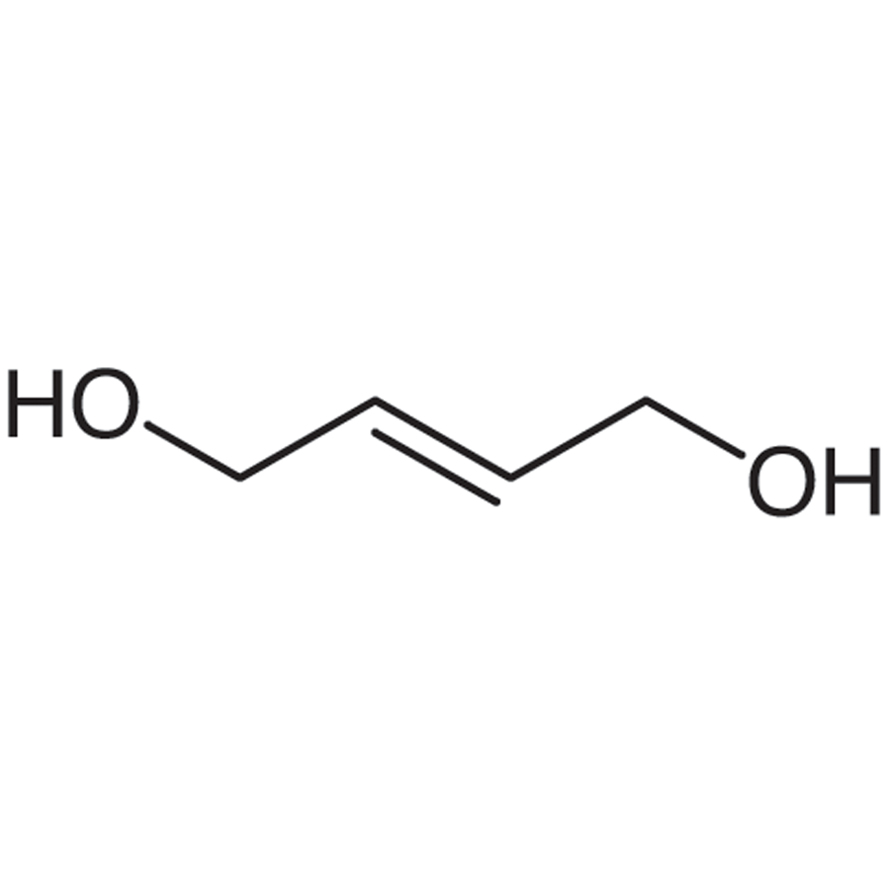
trans2Butene1,4diol 3BB3384 CymitQuimica
Thermodynamics data from equilibrium studies of positional and geometrical isomerization of 1-butene and 2-butene, J. Am. Chem. Soc., 1964, 86, 5416-5420. [ all data ] Levanova and Andreevskii, 1964